FIGURE 2:
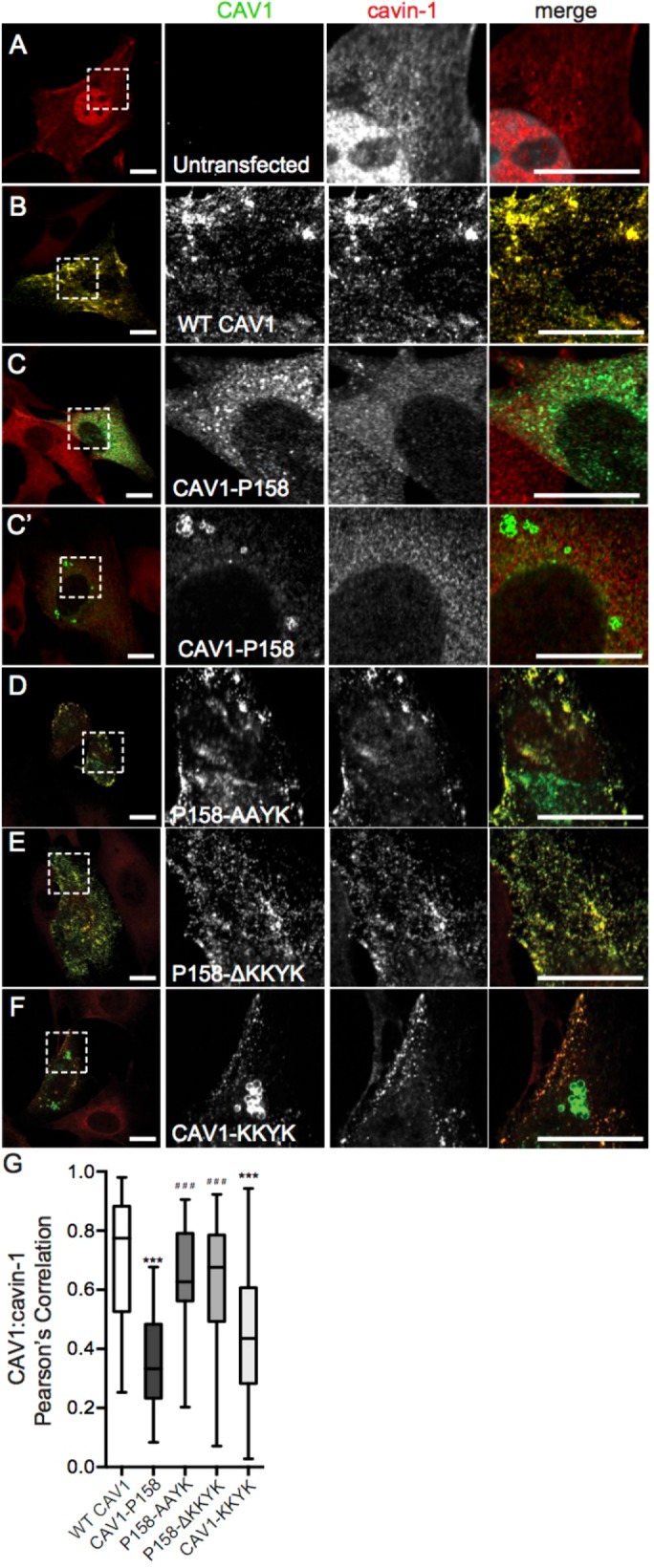
Disruption of the KKYK motif of CAV1-P158 enables the protein to traffic to caveolae. (A) Endogenous cavin-1 immunofluorescence in Cav1–/– MEFs costained with a CAV1 antibody. Note the diffuse distribution of cavin-1 in the absence of Cav1 expression. (B–F) Endogenous cavin-1 immunofluorescence in transfected Cav1–/– MEFs. (B) WT CAV1 colocalizes with endogenous cavin-1 in Cav1–/– MEFs. (C, C′) CAV1-P158 fails to colocalize with cavin-1 and is instead distributed in a diffuse/reticular pattern (C) and/or localizes to vesicular structures with open lumens (C′). (D) CAV1-P158-AAYK colocalizes with cavin-1. (E) CAV1-P158Δ-KKYK also colocalizes with cavin-1. (F) CAV1-KKYK partially colocalizes with cavin-1 but is also found in lipid droplets and/or the ER (also see Figure 1). (G) Quantification of the extent of colocalization between cavin-1 and CAV1 constructs as calculated by Pearson’s correlation. Data are averaged over two to three independent experiments for the following numbers of ROIs: WT CAV1, n = 38; CAV1-P158, n = 40; CAV1-P158-AAYK, n = 52; CAV1-P158Δ-KKYK, n = 46; and CAV1-KKYK, n = 52. Kluskal–Wallis nonparametric ANOVA and a post hoc Dunn’s multiple comparisons test were performed to determine p values. Asterisks (*) and hashtags (#) indicate statistically significant differences compared with wild-type CAV1 and CAV1-P158, respectively. ***/###, p < 0.001. Scale bars = 10 μm.
