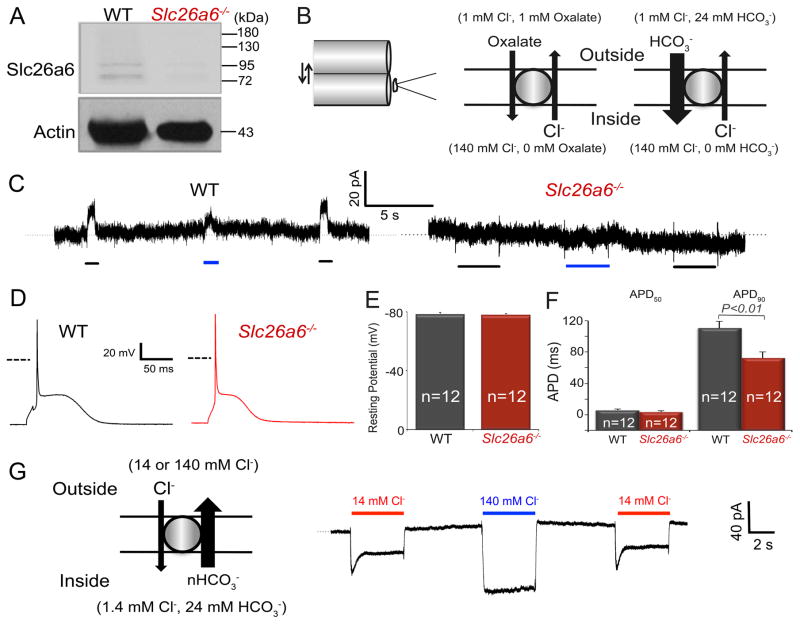
Figure 1. Ablation of Slc26a6−/− results in cardiac AP shortening.
A. Western blot analysis confirms the absence of Slc26a6 expression in Slc26a6−/− mouse hearts. B. Schematic diagram of the fast solution exchange of the suspended whole-cell recording (left), and the ionic configuration to activate outward exchange current through Slc26a6. For recordings of the outward Slc26a6 currents, cardiomyocytes were first clamped at 0 mV, suspended, and moved to the outlet of the solution exchange capillary tube dispensing the bath control solution. Then the myocyte was switched to the capillary tube dispensing bath test solution, containing oxalate or HCO3−, for the activation of outward currents. The fast solution switch was performed by SF-77 solution exchanger controlled by pClamp10 software. The compositions of the bath control and test solutions have been described in Methods. C. Representative recording traces of outward currents recorded from WT ventricular myocytes (left) and Slc26a6−/− ventricular myocytes (right). The black and blue bars under the current traces represent the solution exchange to bath test solutions to activate Cl−/Oxalate and Cl−/HCO3− exchange currents, respectively. D. Representative traces of action potentials (APs) from WT and Slc26a6−/− ventricular myocytes. Dashed lines represent 0 mV. E. Comparisons of resting membrane potentials (RMPs). F. Summary data of the action potential durations at 50% (APD50) and 90% repolarization (APD90). The AP and RMP were recorded in HCO3− buffered bath solution. G. Inward Cl−/HCO3− exchange current elicited from WT ventricular myocytes using fast solution exchange, as indicated in the diagram on the left. The red and blue bars above the current traces represent the solution exchange to bath test solutions containing 14 or 140 mM Cl− to activate Cl−/HCO3− exchange currents, respectively.