Abstract
Periodontitis is characterized by PMN infiltration and formation of neutrophil extracellular traps (NETs). However, their functional role for periodontal health remains complex and partially understood. The main function of NETs appears to be evacuation of dental plaque pathogen-associated molecular patterns. The inability to produce NETs is concomitant with aggressive periodontitis. But in cases with exaggerated NET production, NETs are unable to maintain periodontal health and bystander damages occur. This pathology can be also demonstrated in animal models using lipopolysaccharide as PMN activator. The progress of periodontitis appears to be a consequence of the formation of gingival pockets obstructing the evacuation of both pathogen-associated and damage-associated molecular patterns, which are responsible for the self-perpetuation of inflammation. Thus, besides the pathogenic effects of the periodontal bacteria, the dysregulation of PMN activation appears to play a main role in the periodontal pathology. Consequently, modulation of PMN activation might be a useful approach to periodontal therapy.
Keywords: neutrophils, lipopolysaccharide, Papillon–Lefèvre syndrome, chronic granulomatous disease, bystander damages, NETosis
Introduction
As in other mucosal infections, the host response to the bacteria in periodontitis is characterised by the mucosal efflux of PMNs (1–3). The PMNs influx into the crevice appears to be the first line of defence against plaque bacteria (4). The crevicular PMNs barely phagocytise (5–8), but abundantly form neutrophil extracellular traps (NETs) (4, 8). NETs are an innate immunity defence mechanism chiefly responsible for preventing the bacterial dissemination (9). They are extracellular web-like fibres generated by activated PMNs and are largely composed of nuclear constituents that disarm and kill bacteria extracellularly. NETs have a DNA backbone, but also contain many bactericidal substances, such as histones, human neutrophil elastase (NE), lysozyme, bactericidal permeability-increasing protein, human peptidoglycan-recognition protein S, and other PMN proteins (9–12). NETs bind Gram-positive as well as Gram-negative bacteria, immobilise them, and thus prevent the colonisation of new host surfaces (9). However, NETs can also be triggered by non-infectious agents (9, 13), placental microparticles (13), and inorganic implants (14) and can be harmful for the host (15–22). The capability of NETs to prevent bacterial spreading or to cause bystander damages makes it difficult to comprehend the role of NETs in periodontitis and their impact on the periodontitis pathology also remains elusive.
Mini-Review
Are NETs Beneficial for Periodontal Health?
Analysing the co-occurrence of periodontitis in patients with both known PMN and NETosis deficiencies may help understand the NET impact of NETs on periodontitis.
Papillon–Lefèvre syndrome (PLS) is an autosomal recessive disorder characterised by palmoplantar keratosis and aggressive periodontitis. PLS results from mutations that inactivate cysteine protease cathepsin C (23), which processes various serine proteases including NE, which is an integral structural part of NETs (24, 25). Patients with PLS are either unable to form NETs or produced them in markedly reduced quantities (26, 27). Likewise, inhibitors of NE proteolytic activity, such as small β-lactam-based, cell-permeable NE inhibitors, block the NET release in neutrophils derived from healthy volunteers (25). In addition, the exogenous human secretory leucocyte protease inhibitor markedly inhibits NET formation in human neutrophils (28). The concomitance of aggressive periodontitis and the inability to form NETs suggest the indispensability of NETs for maintaining periodontal health (Figure 1A). Similarly, mutations in ELANE gene encoding NE are associated with aggressive periodontitis in the majority of patients with such mutations (29). Quite recently, the inability to form NETs has been reported for ELANE mutations (30).
Figure 1.
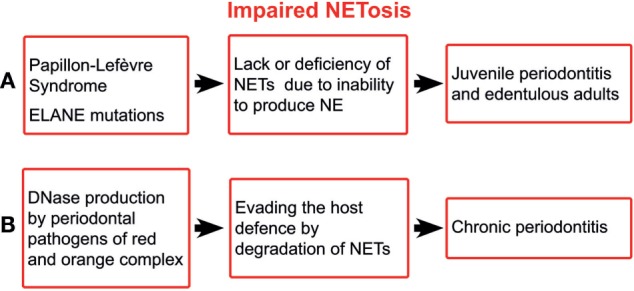
Schematic illustration of impaired NETosis in periodontitis. ELANE, the gene encoding neutrophil elastase; NETs, neutrophil extracellular traps; NE, neutrophil elastase.
Chronic granulomatous disease (CGD) is a rare primary immunodeficiency affecting the innate immune system, caused by mutations in any one of four genes encoding the subunits of the superoxide generating phagocyte NADPH oxidase, resulting in an absence or very low levels of enzyme activity (31). However, periodontitis appears to be occasional in CGD patients. Only isolated cases of periodontitis have been reported in CGD patients (32–34). A survey on 368 CGD patients reported merely nine cases of gingivitis or periodontitis (35). Individuals with inherited deficient NADPH oxidase activity, i.e., CGD patients, are capable of inducing NETosis via a NADPH oxidase-independent pathway; either via an ROS-dependent mechanism utilising ROS from other sources (36) or an ROS-independent mechanism (37). Many trigger mechanisms could be responsible for NADPH oxidase-independent NETosis in CGD patients. Thus, NADPH-oxidase-independent NETosis is stimulated by higher doses of hepoxilin A3 (38). Another possibility of CGD PMNs to produce NETs in the crevice is to utilise mitochondrial ROS (39), or other sources, e.g., ROS produced by plaque bacteria as Streptococcus sanguinis and Streptococcus oralis (40, 41). Further, Candida albicans (42) triggers ROS-independent NETosis as well as Staphylococcus aureus ROS-independent (43) and oxidant-independent NETosis (44). The fact that CGD patients are not disposed to periodontitis suggests that the oxidative burst does not appear to play a crucial role in maintaining the periodontal health, but NETs constitute the main defence. The main function of NETs appears to be that of shielding the gingiva and clearing bacteria, and their metabolic products, out of the crevice.
The ability of the major periodontal pathogens, i.e., those of red and orange complex, to produce deoxyribonucleases (45) suggests the importance of NETs for the host defence. It has been shown that extracellular nucleases enable periodontal pathogens to degrade the host NETs, leading to increased pathogenicity (46) (Figure 1B). Although the bacterial nucleases do not affect the NET proteases, the latter alone are not able to provide sufficient protection against periodontal pathogens.
The inability of patients with PLS and most of those with ELANE mutations to form NETs is concomitant with aggressive periodontitis. The ability of CGD patients to form oxidase-independent NETs is a possible explanation for the rarity of periodontitis in these patients. The most aggressive periodontal pathogens produce DNases to degrade NETs. In sum, the NET deficiency paired with aggressive periodontitis indicates the indispensability of NET for maintaining the periodontal health.
Can NETs Be Harmful in Periodontitis?
The lipopolysaccharide (LPS) component of the cell wall of Gram-negative bacteria is an important pathogen-associated molecular pattern (PAMP) that triggers an innate immune response mainly through the activation of the toll-like receptor 4. LPS is a potent inducer of NETs (9). The supernatant of dental plaque also triggers NETosis (47). Even elevated blood plasma LPS levels have been registered in aggressive periodontitis (48) (Figure 2A). A LPS injection into the gingival tissues is a model for examining how the innate immune response to this bacterial component induces experimental periodontitis (49, 50). Histopathologically, this model is similar to other periodontitis models and to the periodontitis in humans, characterised by increased infiltration of leucocytes, higher levels of pro-inflammatory cytokines, collagen degradation, and alveolar bone resorption. Typically, a defined amount of purified bacterial LPS suspended into small micro-volumes (1–6 µl) is injected into the gingival tissues surrounding the posterior teeth of either mice or rats (51). LPS and other plaque PAMPs as well as damage-associated molecular patterns (DAMPs) activate the endothelial cells (ECs), due to the insignificant distance between high endothelial venules (HEVs) and the crevice (52, 53). Alveolar bone loss has been induced by injections of LPS from various microorganisms, including Escherichia coli, Aggregatibacter actinomycetemcomitans, and Salmonella typhimurium (51). LPS-activated ECs become leaky, as shown in the acute lung injury (54), and trigger PMN transmigration. After transmigration across the HEVs, PMNs are attracted to the crevice by PAMPs and DAMPs. LPS-stimulated PMNs selectively secrete IL8, MIP1β, and TNFα (55), which maintain EC activation. Thus, a vicious circle of PMN/HEV mutual paracrine activation may yield an exaggerated PMN response damaging the periodontal tissues. Unquestionably, the LPS effect is not restricted to HEV and PMN activations but affects the entire immunity. Thus, PMN infiltration of gingiva, PMN influx into the crevice, and subsequent NETosis is a crucial feature of periodontitis, which is an exaggerated response to the non-infectious LPS challenge. Nonetheless, PMN efflux cannot be separated from the capillaries and neither can NETosis from the PMN activation, as NETs are just a developmental stage of PMNs. The lack of resolution signals warrants the periodontal inflammation (56). The systemic effects of NETs in periodontal disease may contribute to the body’s overall inflammatory burden, worsening conditions such as diabetes mellitus, obstructive pulmonary disease, and atherosclerosis (56–60). Further, periodontitis-derived citrullinated histones (8, 61) may trigger autoimmunity, especially in rheumatoid arthritis.
Figure 2.
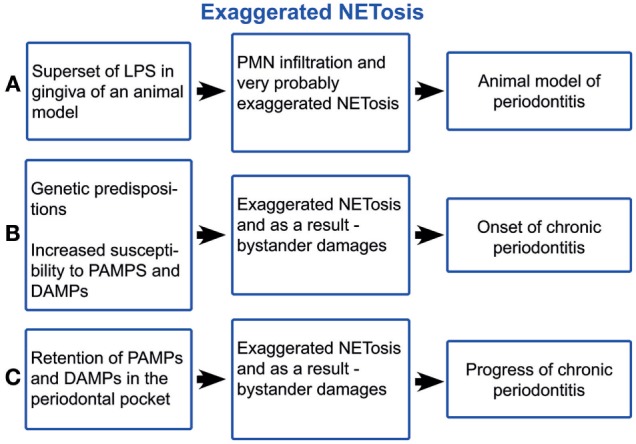
Schematic illustration of exaggerated NETosis in periodontitis. LPS, lipopolysaccharide; PMNs, polymorphonuclear neutrophil leukocytes; PAMPs, pathogen-associated molecular pattern; DAMPs, damage-associated molecular patterns.
What Underlies NET Dysregulation in Periodontitis?
Genetic predispositions appear to be crucial for both the onset and the progression of periodontitis (62, 63). Chronic periodontitis occurs when untreated gingivitis progresses to the loss of the gingiva, bone, and ligament, which creates the deep periodontal “pockets” that are a hallmark of the disease (63). The pocket extends the evacuation route of the crevicular fluid, which is the blood ultra-filtrate continuously secreted in the periodontal crevice (64). NETs form a three-dimensional network entangling the particles within the crevice, notably disseminated bacteria, desquamated epithelial cells, cell debris, and fragments of biofilm matrix (4). This network is flushed out by the crevicular fluid outflow. Concomitantly with deepening the periodontal pocket, morphological changes of the pocket epithelium take place, primarily the inflammatory papillary hyperplasia. As a result, many narrow chasms between the papillae are formed, they are filled with partially and completely exfoliated epithelial cells, which cannot be efficiently flushed out by the crevicular fluid outflow (65), i.e., the pocket obstructs the evacuation of PAMPs and DAMPs out of the crevice (Figure 2B). The exaggerated NET formation causes viscosity rise (66, 67) of crevicular fluid and as a result obstruction of PAMP and DAMP evacuation. Further, NET formation is directly induced by many oral bacteria from the dental plaque (41, 47, 68, 69), neutrophil pro-inflammatory chemokines (9, 13, 70), and neutrophil-produced ROS (24). After surgery (71, 72), healing is achieved through the formation of a long junctional epithelium or a new connective tissue attachment to the previously diseased root surface, i.e., through removing the pocket obstruction of the PAMP and DAPM clearing. Thus, periodontitis occurs, given genetic susceptibility (62, 63), as consequence of the exaggerated host response to PAMP and DAPM, as the case of experimental LPS-induced periodontitis is (Figure 2C). This self-perpetuating periodontal inflammation has many common characteristics with the chronic obstructive pulmonary disease. Both diseases are characterised by heavy PMN infiltration and NETosis (73, 74), obstruction of PAMP, and DAMP evacuation and aggravation through smoking, as cigarette smoke induces NETs (75).
In cases with exaggerated production of NETs, modulation of PMN activation and NET triggering might be a helpful approach for periodontitis treatment. A broad spectrum of antioxidative substances such as flavonoids, vitamin C, 5-aminosalicylic acid, and N-acetyl-l-cysteine significantly inhibit the formation of ROS-dependent NETs (76). In addition, LPS effects can be reduced by gallic acid and thereby also NETosis (77). In view of the fact that some of these substances are innoxious, they might be applied topically, e.g., as dentifrice or in cases of exacerbations instilled into periodontal pockets. Indeed, further investigations are needed to estimate such possibilities.
Conclusion
The inability of patients with PLS and most of those with ELANE mutations to form NETs indicates the role of NETs for maintaining periodontal health. The periodontal pocket formation causes clearance obstruction of PAMPs and DAMPs. The sustained PAMP and DAMP challenge triggers the exaggerated NETosis, which causes bystander damages and the disease progress. Once formed, the periodontal pocket boosts the progress of periodontitis. Modulation of exaggerated NET production by topical application of NET inhibitors might be a possible approach for prevention and treatment of periodontitis.
Author Contributions
LV designed the study and drafted the manuscript. LV, DH, BM, and MH critically commented on the paper, contributed towards and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Attström R, Egelberg J. Emigration of blood neutrophils and monocytes into the gingival crevices. J Periodontal Res (1970) 5:48–55. 10.1111/j.1600-0765.1970.tb01837.x [DOI] [PubMed] [Google Scholar]
- 2.Kinane D, Berglundh T, Lindhe J. Host–parasite interactions in periodontal disease. In: Lindhe J, Karring T, Lang N, editors. Clinical Periodontology and Implant Dentistry. Copenhagen: Blackwell Munksgaard; (2015). 1480 p. [Google Scholar]
- 3.Armitage GC. Clinical evaluation of periodontal diseases. Periodontol 2000 (1995) 7:39–53. 10.1111/j.1600-0757.1995.tb00035.x [DOI] [PubMed] [Google Scholar]
- 4.Vitkov L, Klappacher M, Hannig M, Krautgartner WD. Extracellular neutrophil traps in periodontitis. J Periodontal Res (2009) 44:664–72. 10.1111/j.1600-0765.2008.01175.x [DOI] [PubMed] [Google Scholar]
- 5.Newman HN, Addison IE. Gingival crevice neutrophil function in periodontosis. J Periodontol (1982) 53:578–86. 10.1902/jop.1982.53.9.578 [DOI] [PubMed] [Google Scholar]
- 6.Sigusch B, Klinger G, Holtz H, Süss J. In vitro phagocytosis by crevicular phagocytes in various forms of periodontitis. J Periodontol (1992) 63:496–501. 10.1902/jop.1992.63.6.496 [DOI] [PubMed] [Google Scholar]
- 7.Miyazaki A, Kobayashi T, Suzuki T, Yoshie H, Hara K. Loss of Fcgamma receptor and impaired phagocytosis of polymorphonuclear leukocytes in gingival crevicular fluid. J Periodontal Res (1997) 32:439–46. 10.1111/j.1600-0765.1997.tb00556.x [DOI] [PubMed] [Google Scholar]
- 8.Vitkov L, Klappacher M, Hannig M, Krautgartner WD. Neutrophil fate in gingival crevicular fluid. Ultrastruct Pathol (2010) 34:25–30. 10.3109/01913120903419989 [DOI] [PubMed] [Google Scholar]
- 9.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science (2004) 303:1532–5. 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 10.Cho JH, Fraser IP, Fukase K, Kusumoto S, Fujimoto Y, Stahl GL, et al. Human peptidoglycan recognition protein S is an effector of neutrophil-mediated innate immunity. Blood (2005) 106:2551–8. 10.1182/blood-2005-02-0530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaillon S, Peri G, Delneste Y, Frémaux I, Doni A, Moalli F, et al. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J Exp Med (2007) 204:793–804. 10.1084/jem.20061301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog (2009) 5:e1000639. 10.1371/journal.ppat.1000639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta AK, Hasler P, Holzgreve W, Gebhardt S, Hahn S. Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum Immunol (2005) 66:1146–54. 10.1016/j.humimm.2005.11.003 [DOI] [PubMed] [Google Scholar]
- 14.Vitkov L, Krautgartner W-D, Obermayer A, Stoiber W, Hannig M, Klappacher M, et al. The initial inflammatory response to bioactive implants is characterized by NETosis. PLoS One (2015) 10:e0121359. 10.1371/journal.pone.0121359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn S, Gupta AK, Troeger C, Rusterholz C, Holzgreve W. Disturbances in placental immunology: ready for therapeutic interventions? Springer Semin Immunopathol (2006) 27:477–93. 10.1007/s00281-006-0016-5 [DOI] [PubMed] [Google Scholar]
- 16.Gupta AK, Hasler P, Holzgreve W, Hahn S. Neutrophil NETs: a novel contributor to preeclampsia-associated placental hypoxia? Semin Immunopathol (2007) 29:163–7. 10.1007/s00281-007-0073-4 [DOI] [PubMed] [Google Scholar]
- 17.Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, Werb Z, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med (2009) 15:623–5. 10.1038/nm.1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hakkim A, Fürnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A (2010) 107:9813–8. 10.1073/pnas.0909927107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest (2012) 122:2661–71. 10.1172/JCI61303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Bruhl M-L, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med (2012) 209:819–35. 10.1084/jem.20112322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahlenberg JM, Carmona-Rivera C, Smith CK, Kaplan MJ. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J Immunol (2013) 190:1217–26. 10.4049/jimmunol.1202388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Säll J, Carlsson M, Gidlöf O, Holm A, Humlén J, Öhman J, et al. The antimicrobial peptide LL-37 alters human osteoblast Ca2+ handling and induces Ca2+-independent apoptosis. J Innate Immun (2013) 5:290–300. 10.1159/000346587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wani AA, Devkar N, Patole MS, Shouche YS. Description of two new cathepsin C gene mutations in patients with Papillon-Lefèvre syndrome. J Periodontol (2006) 77:233–7. 10.1902/jop.2006.050124 [DOI] [PubMed] [Google Scholar]
- 24.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol (2007) 176:231–41. 10.1083/jcb.200606027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol (2010) 191:677–91. 10.1083/jcb.201006052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sørensen OE, Clemmensen SN, Dahl SL, Østergaard O, Heegaard NH, Glenthøj A, et al. Papillon-Lefèvre syndrome patient reveals species-dependent requirements for neutrophil defenses. J Clin Invest (2014) 124:4539–48. 10.1172/JCI76009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts H, White P, Dias I, McKaig S, Veeramachaneni R, Thakker N, et al. Characterization of neutrophil function in Papillon-Lefevre syndrome. J Leukoc Biol (2016) 100:433–44. 10.1189/jlb.5A1015-489R [DOI] [PubMed] [Google Scholar]
- 28.Zabieglo K, Majewski P, Majchrzak-Gorecka M, Wlodarczyk A, Grygier B, Zegar A, et al. The inhibitory effect of secretory leukocyte protease inhibitor (SLPI) on formation of neutrophil extracellular traps. J Leukoc Biol (2015) 98:99–106. 10.1189/jlb.4AB1114-543R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye Y, Carlsson G, Wondimu B, Fahlén A, Karlsson-Sjöberg J, Andersson M, et al. Mutations in the ELANE gene are associated with development of periodontitis in patients with severe congenital neutropenia. J Clin Immunol (2011) 31:936–45. 10.1007/s10875-011-9572-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thanarajasingam U, Jensen MA, Dorschner JM, Wampler Muskardin T, Ghodke-Puranik Y, Purmalek M, et al. A novel ELANE mutation associated with inflammatory arthritis, defective NETosis, and recurrent parvoviral infection. Arthritis Rheumatol (2017). 10.1002/art.40314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heyworth PG, Cross AR, Curnutte JT. Chronic granulomatous disease. Curr Opin Immunol (2003) 15:578–84. 10.1016/S0952-7915(03)00109-2 [DOI] [PubMed] [Google Scholar]
- 32.Cohen MS, Leong PA, Simpson DM. Phagocytic cells in periodontal defense: periodontal status of patients with chronic granulomatous disease of childhood. J Periodontol (1985) 56:611–7. 10.1902/jop.1985.56.10.611 [DOI] [PubMed] [Google Scholar]
- 33.Buduneli N, Baylas H, Aksu G, Kütükçüler N. Prepubertal periodontitis associated with chronic granulomatous disease. J Clin Periodontol (2001) 28:589–93. 10.1034/j.1600-051x.2001.028006589.x [DOI] [PubMed] [Google Scholar]
- 34.Dar-Odeh NS, Hayajneh WA, Abu-Hammad OA, Hammad HM, Al-Wahadneh AM, Bulos NK, et al. Orofacial findings in chronic granulomatous disease: report of twelve patients and review of the literature. BMC Res Notes (2010) 3:37. 10.1186/1756-0500-3-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winkelstein JA, Marino MC, Johnston RB, Boyle J, Curnutte J, Gallin JI, et al. Chronic granulomatous disease: report on a national registry of 368 patients. Medicine (2000) 79:155–69. 10.1097/00005792-200005000-00003 [DOI] [PubMed] [Google Scholar]
- 36.Nishinaka Y, Arai T, Adachi S, Takaori-Kondo A, Yamashita K. Singlet oxygen is essential for neutrophil extracellular trap formation. Biochem Biophys Res Commun (2011) 413:75–9. 10.1016/j.bbrc.2011.08.052 [DOI] [PubMed] [Google Scholar]
- 37.Arai Y, Nishinaka Y, Arai T, Morita M, Mizugishi K, Adachi S, et al. Uric acid induces NADPH oxidase-independent neutrophil extracellular trap formation. Biochem Biophys Res Commun (2014) 443:556–61. 10.1016/j.bbrc.2013.12.007 [DOI] [PubMed] [Google Scholar]
- 38.Douda DN, Grasemann H, Pace-Asciak C, Palaniyar NA. Lipid mediator hepoxilin A3 is a natural inducer of neutrophil extracellular traps in human neutrophils. Mediators Inflamm (2015) 2015:1–7. 10.1155/2015/520871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Douda DN, Khan MA, Grasemann H, Palaniyar N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc Natl Acad Sci U S A (2015) 112:2817–22. 10.1073/pnas.1414055112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okahashi N, Sumitomo T, Nakata M, Sakurai A, Kuwata H, Kawabata S. Hydrogen peroxide contributes to the epithelial cell death induced by the oral mitis group of streptococci. PLoS One (2014) 9:e88136. 10.1371/journal.pone.0088136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sumioka R, Nakata M, Okahashi N, Li Y, Wada S, Yamaguchi M, et al. Streptococcus sanguinis induces neutrophil cell death by production of hydrogen peroxide. PLoS One (2017) 12:e0172223. 10.1371/journal.pone.0172223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Byrd AS, O’Brien XM, Johnson CM, Lavigne LM, Reichner JS. An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to Candida albicans. J Immunol (2013) 190:4136–48. 10.4049/jimmunol.1202671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Björnsdottir H, Dahlstrand Rudin A, Klose FP, Elmwall J, Welin A, Stylianou M, et al. Phenol-soluble modulin α peptide toxins from aggressive Staphylococcus aureus induce rapid formation of neutrophil extracellular traps through a reactive oxygen species-independent pathway. Front Immunol (2017) 8:257. 10.3389/fimmu.2017.00257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pilsczek FH, Salina D, Poon KKH, Fahey C, Yipp BG, Sibley CD, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol (2010) 185:7413–25. 10.4049/jimmunol.1000675 [DOI] [PubMed] [Google Scholar]
- 45.Palmer LJ, Chapple ILC, Wright HJ, Roberts A, Cooper PR. Extracellular deoxyribonuclease production by periodontal bacteria: deoxyribonuclease production by periodontal bacteria. J Periodontal Res (2012) 47:439–45. 10.1111/j.1600-0765.2011.01451.x [DOI] [PubMed] [Google Scholar]
- 46.Doke M, Fukamachi H, Morisaki H, Arimoto T, Kataoka H, Kuwata H. Nucleases from Prevotella intermedia can degrade neutrophil extracellular traps. Mol Oral Microbiol (2017) 32:288–300. 10.1111/omi.12171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirschfeld J, Dommisch H, Skora P, Horvath G, Latz E, Hoerauf A, et al. Neutrophil extracellular trap formation in supragingival biofilms. Int J Med Microbiol (2015) 305:453–63. 10.1016/j.ijmm.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 48.Shaddox LM, Wiedey J, Calderon NL, Magnusson I, Bimstein E, Bidwell JA, et al. Local inflammatory markers and systemic endotoxin in aggressive periodontitis. J Dent Res (2011) 90:1140–4. 10.1177/0022034511413928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abe T, Hajishengallis G. Optimization of the ligature-induced periodontitis model in mice. J Immunol Methods (2013) 394:49–54. 10.1016/j.jim.2013.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nogueira AVB, Chaves de Souza JA, de Molon RS, da Silva Mariano Pereira E, de Aquino SG, Giannobile WV, et al. HMGB1 localization during experimental periodontitis. Mediators Inflamm (2014) 2014:1–10. 10.1155/2014/816320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Graves DT, Kang J, Andriankaja O, Wada K, Rossa C., Jr Animal models to study host-bacteria interactions involved in periodontitis. In: Kinane DF, Mombelli A, editors. Frontiers of Oral Biology. Basel: KARGER; (2012). p. 117–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zoellner H, Chapple CC, Hunter N. Microvasculature in gingivitis and chronic periodontitis: disruption of vascular networks with protracted inflammation. Microsc Res Tech (2002) 56:15–31. 10.1002/jemt.10009 [DOI] [PubMed] [Google Scholar]
- 53.Kasprzak A, Surdacka A, Tomczak M, Konkol M. Role of high endothelial postcapillary venules and selected adhesion molecules in periodontal diseases: a review: high endothelial venules in periodontitis. J Periodontal Res (2013) 48:1–21. 10.1111/j.1600-0765.2012.01492.x [DOI] [PubMed] [Google Scholar]
- 54.Gandhirajan RK, Meng S, Chandramoorthy HC, Mallilankaraman K, Mancarella S, Gao H, et al. Blockade of NOX2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. J Clin Invest (2013) 123(2):887–902. 10.1172/JCI65647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Dyke TE. Pro-resolving mediators in the regulation of periodontal disease. Mol Aspects Med (2017). 10.1016/j.mam.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jeffcoat MK, Hauth JC, Geurs NC, Reddy MS, Cliver SP, Hodgkins PM, et al. Periodontal disease and preterm birth: results of a pilot intervention study. J Periodontol (2003) 74:1214–8. 10.1902/jop.2003.74.8.1214 [DOI] [PubMed] [Google Scholar]
- 57.Khader YS, Dauod AS, El-Qaderi SS, Alkafajei A, Batayha WQ. Periodontal status of diabetics compared with nondiabetics: a meta-analysis. J Diabetes Complications (2006) 20:59–68. 10.1016/j.jdiacomp.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 58.Gotsman I, Lotan C, Soskolne WA, Rassovsky S, Pugatsch T, Lapidus L, et al. Periodontal destruction is associated with coronary artery disease and periodontal infection with acute coronary syndrome. J Periodontol (2007) 78:849–58. 10.1902/jop.2007.060301 [DOI] [PubMed] [Google Scholar]
- 59.Kucukcoskun M, Baser U, Oztekin G, Kiyan E, Yalcin F. Initial periodontal treatment for prevention of chronic obstructive pulmonary disease exacerbations. J Periodontol (2013) 84:863–70. 10.1902/jop.2012.120399 [DOI] [PubMed] [Google Scholar]
- 60.Hobbins S, Chapple I, Sapey E, Stockley R. Is periodontitis a comorbidity of COPD or can associations be explained by shared risk factors/behaviors? Int J Chron Obstruct Pulmon Dis (2017) 12:1339–49. 10.2147/COPD.S127802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Janssen KMJ, de Smit MJ, Withaar C, Brouwer E, van Winkelhoff AJ, Vissink A, et al. Autoantibodies against citrullinated histone H3 in rheumatoid arthritis and periodontitis patients. J Clin Periodontol (2017) 44:577–84. 10.1111/jcpe.12727 [DOI] [PubMed] [Google Scholar]
- 62.Borrell LN, Papapanou PN. Analytical epidemiology of periodontitis. J Clin Periodontol (2005) 32:132–58. 10.1111/j.1600-051X.2005.00799.x [DOI] [PubMed] [Google Scholar]
- 63.Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primer (2017) 3:17038. 10.1038/nrdp.2017.38 [DOI] [PubMed] [Google Scholar]
- 64.Goodson JM. Gingival crevice fluid flow. Periodontol 2000 (2003) 31:43–54. 10.1034/j.1600-0757.2003.03104.x [DOI] [PubMed] [Google Scholar]
- 65.Vitkov L, Krautgartner WD, Hannig M. Surface morphology of pocket epithelium. Ultrastruct Pathol (2005) 29:121–7. 10.1080/01913120590916832 [DOI] [PubMed] [Google Scholar]
- 66.Picot R, Das I, Reid L. Pus, deoxyribonucleic acid, and sputum viscosity. Thorax (1978) 33:235–42. 10.1136/thx.33.2.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shak S, Capon DJ, Hellmiss R, Marsters SA, Baker CL. Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. Proc Natl Acad Sci U S A (1990) 87:9188–92. 10.1073/pnas.87.23.9188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palmer LJ, Damgaard C, Holmstrup P, Nielsen CH. Influence of complement on neutrophil extracellular trap release induced by bacteria. J Periodontal Res (2016) 51:70–6. 10.1111/jre.12284 [DOI] [PubMed] [Google Scholar]
- 69.Hirschfeld J, Roberts HM, Chapple ILC, Parčina M, Jepsen S, Johansson A, et al. Effects of Aggregatibacter actinomycetemcomitans leukotoxin on neutrophil migration and extracellular trap formation. J Oral Microbiol (2016) 8:33070. 10.3402/jom.v8.33070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alfaro C, Teijeira A, Onate C, Perez G, Sanmamed MF, Andueza MP, et al. Tumor-produced interleukin-8 attracts human myeloid-derived suppressor cells and elicits extrusion of neutrophil extracellular traps (NETs). Clin Cancer Res (2016) 22:3924–36. 10.1158/1078-0432.CCR-15-2463 [DOI] [PubMed] [Google Scholar]
- 71.Everett FG, Waerhaug J, Widman A. Leonard Widman: surgical treatment of pyorrhea alveolaris. J Periodontol (1971) 42:571–9. 10.1902/jop.1971.42.9.571 [DOI] [PubMed] [Google Scholar]
- 72.Ramfjord SP, Nissle RR. The modified Widman flap. J Periodontol (1974) 45:601–7. 10.1902/jop.1974.45.8.2.601 [DOI] [PubMed] [Google Scholar]
- 73.Qiu Y, Zhu J, Bandi V, Atmar RL, Hattotuwa K, Guntupalli KK, et al. Biopsy neutrophilia, neutrophil chemokine and receptor gene expression in severe exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med (2003) 168:968–75. 10.1164/rccm.200208-794OC [DOI] [PubMed] [Google Scholar]
- 74.Obermayer A, Stoiber W, Krautgartner W-D, Klappacher M, Kofler B, Steinbacher P, et al. New aspects on the structure of neutrophil extracellular traps from chronic obstructive pulmonary disease and in vitro generation. PLoS One (2014) 9:e97784. 10.1371/journal.pone.0097784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qiu S-L, Zhang H, Tang Q, Bai J, He Z-Y, Zhang J-Q, et al. Neutrophil extracellular traps induced by cigarette smoke activate plasmacytoid dendritic cells. Thorax (2017). 10.1136/thoraxjnl-2016-209887 [DOI] [PubMed] [Google Scholar]
- 76.Kirchner T, Hermann E, Möller S, Klinger M, Solbach W, Laskay T, et al. Flavonoids and 5-aminosalicylic acid inhibit the formation of neutrophil extracellular traps. Mediators Inflamm (2013) 2013:1–14. 10.1155/2013/710239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haute GV, Caberlon E, Squizani E, de Mesquita FC, Pedrazza L, Martha BA, et al. Gallic acid reduces the effect of LPS on apoptosis and inhibits the formation of neutrophil extracellular traps. Toxicol In Vitro (2015) 30:309–17. 10.1016/j.tiv.2015.10.005 [DOI] [PubMed] [Google Scholar]


