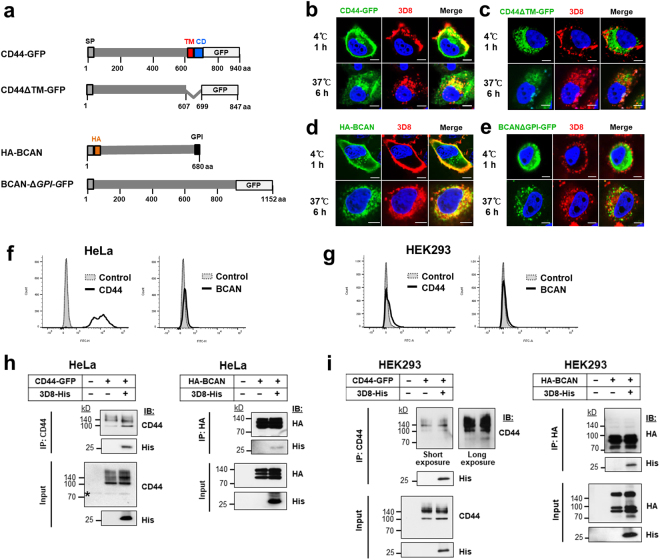
Figure 4.
CD44 and BCAN, representative CSPGs, act as endocytic receptors for 3D8 scFv on HeLa and HEK293 cells. (a) Schematic diagrams of CD44 and BCAN, which are expressed on the cell surface, and their recombinants, which are expressed intracellularly (controls). SP, signal peptide; Δ, deletion; TM, transmembrane domain; CD, cytoplasmic domain; GPI, GPI anchor; GFP, green fluorescent protein; HA, HA tag. (b–e) Confocal microscopy. HeLa cells were transfected with plasmids encoding CD44-GFP (b) and HA-BCAN (d), which are expressed on the cell surface, or with plasmids encoding CD44ΔTM-GFP (c) and BCAN-ΔGPI-GFP (e), which are expressed intracellularly. At 18 h post-transfection, cells were incubated with 10 μM 3D8 scFv under the specified conditions. After fixation and permeabilization, cells transfected with plasmids containing the GFP gene were incubated with a rabbit anti-3D8 scFv antibody, followed by a TRITC-conjugated goat anti-rabbit IgG antibody. Cells transfected with the plasmid encoding the HA-BCAN gene were incubated with a rabbit anti-3D8 scFv antibody and a mouse anti-HA antibody, which were detected by TRITC-conjugated goat anti-rabbit IgG and Alexa Fluor 488-conjugated goat anti-mouse IgG, respectively. Nuclei were stained with Hoechst 33342 (blue). Bar, 10 μm. (f,g) Cell surface expression of endogenous CD44 and BCAN was examined by flow cytometry. (h,i) Co-immunoprecipitation. HeLa (h) and HEK293 cells (i) were transfected with plasmids encoding CD44-GFP and HA-BCAN and 24 h later treated with 5 μM 3D8 scFv for 6 h at 37 °C. Cells were collected and lysed for co-immunoprecipitation assays. Co-immunoprecipitation was performed using an anti-GFP antibody, Samples were analyzed by immunoblotting with antibodies specific for CD44 and the His tag (left panels of h, i). Samples from the co-immunoprecipitation performed with the anti-HA antibody, were analyzed by immunoblotting with antibodies specific for the HA tag and the His tag (right panels of h, i). Cells not treated with 3D8 scFv protein were used as a negative control. Proteins from the extract (Input; 10%) and pulled-down fractions (IP) were analyzed by immunoblotting. Asterisks denote endogenous CD44.