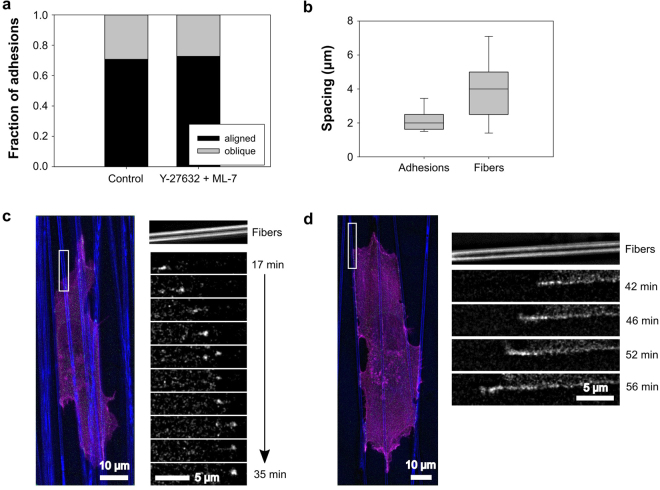
Figure 5.
Aligned fibers provide a continuous substrate for the sequential formation of adhesions. (a) Fraction of new adhesions associated with protrusions aligned or oblique to the predominant fiber orientation on aligned PCL scaffolds in the presence of absence of 20 µM Y-27632 and 10 µM ML-7. Distribution of adhesions on aligned vs. oblique fibers is essentially unchanged under MII inhibition. (b) HT-1080 cell under MII inhibition, cultured on an aligned PCL substrate (blue) and expressing EGFP-paxillin (green) and Ruby-Lifeact (actin; magenta). Inset: Small, transient adhesions form sequentially as a protrusion progresses along a fiber (inset is rotated 90 degrees clockwise with respect to the color image). (c) Distribution of spacing between newly formed adhesions in HT-1080 cells under MII inhibition and between fibers in aligned PCL scaffolds (in the direction perpendicular to fiber alignment). Average spacing between PCL fibers is significantly larger than average spacing between sequentially formed adhesions (p < 0.01; Kruskal-Wallis test; n = 20 adhesions, 67 fibers). (d) MII-inhibited HT-1080 imaged under conditions similar to panel B. Inset: Adhesion appears to grow from distal end (left side), likely due to the sequential formation of closely spaced sub-diffraction adhesions as the protrusion advances along the fiber (inset is rotated 90 degrees counter-clockwise with respect to the color image). Images in panels B and D are representative of observations made in seven independent experiments. Data are from same experiments as Fig. 4. Times indicated in the figure are relative to the time of seeding. All images are maximum projections of z-stacks. Paxillin images have been additionally processed (“flattened”) to reduce background noise (see Materials and Methods). Scale bars, 10 µm (main panels), 5 µm (insets).