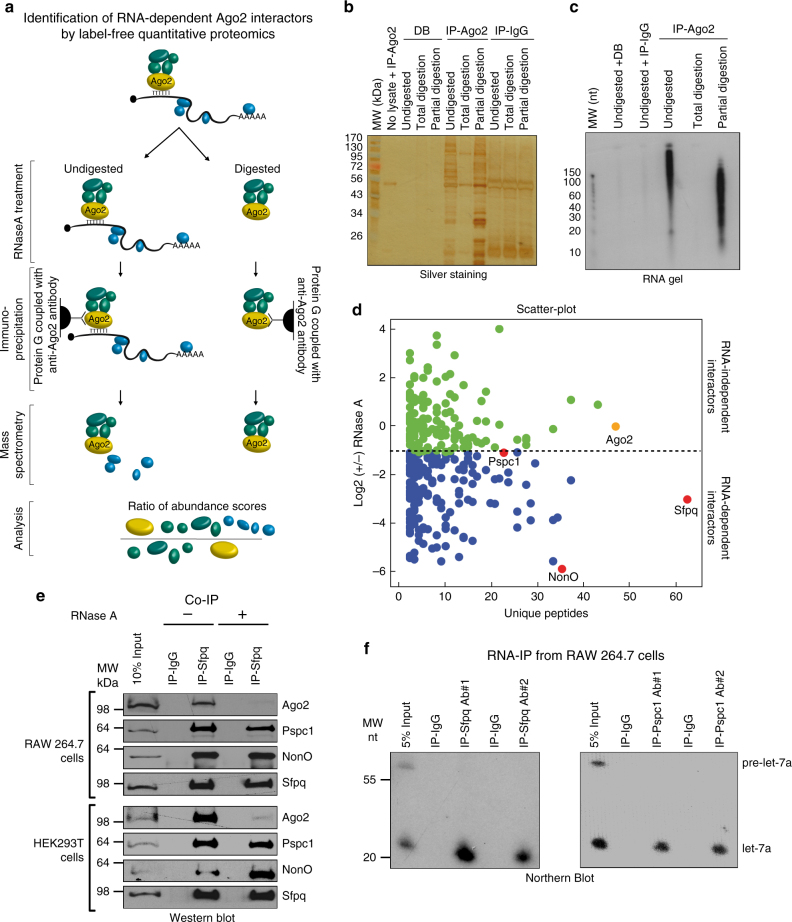
Fig. 1.
Sfpq, Pspc1, and NonO are components of Ago2 complex and interact with let-7a. a Overview of the proteomic method used to identify RNA-dependent proteins interacting with Ago2. b Silver staining of an SDS-PAGE analysis from the IP with the anti-Ago2 antibody, the protein G conjugated with the Dynabeads (DB), or the anti-Ago2 antibody alone incubated without cell lysate. The samples were untreated, treated with 10 μg ml−1 RNase A for 30 min at room temperature (for partial digestion), or treated with 10 mg ml−1 RNase A for 30 min at room temperature (for total digestion). c Radioactive images of a TBE-Urea gel showing signal from 32P-labeled RNA fragments of samples untreated, treated with 10 μg ml−1 RNase A for 30 min at room temperature (for partial digestion), or treated with 10 mg ml−1 RNase A for 30 min at room temperature (for total digestion). d Scatter-plot of the log base 2 (−/+) RNase A ratios (abundance scores) plotted with the unique peptides for each identified protein. Each spot is a different protein. e Co-IP of endogenous Sfpq and Ago2, Pspc1, or NonO in RAW 264.7 and HEK293T cells. When indicated, cell lysates were incubated at room temperature with RNase A (10 mg ml−1) for 30 min. f Sfpq and Pspc1 interact with mature let-7a but not with the precursor. RAW 264.7 cell extracts were immunoprecipitated with two different antibodies for each indicated protein. The RNA was purified and analyzed by Northern blotting