Abstract
Background
Cofactor engineering is involved in the modification of enzymes related to nicotinamide adenine dinucleotides (NADH and NAD+) metabolism, which results in a significantly altered spectrum of metabolic products. Cofactor engineering plays an important role in metabolic engineering but is rarely reported in the sterols biotransformation process owing to its use of multi-catabolic enzymes, which promote multiple consecutive reactions. Androst-4-ene-3, 17-dione (AD) and androst-1, 4-diene-3, 17-dione (ADD) are important steroid medicine intermediates that are obtained via the nucleus oxidation and the side chain degradation of phytosterols by Mycobacterium. Given that the biotransformation from phytosterols to AD (D) is supposed to be a NAD+-dependent process, this work utilized cofactor engineering in Mycobacterium neoaurum and investigated the effect on cofactor and phytosterols metabolism.
Results
Through the addition of the coenzyme precursor of nicotinic acid in the phytosterols fermentation system, the intracellular NAD+/NADH ratio and the AD (D) production of M. neoaurum TCCC 11978 (MNR M3) were higher than in the control. Moreover, the NADH: flavin oxidoreductase was identified and was supposed to exert a positive effect on cofactor regulation and phytosterols metabolism pathways via comparative proteomic profiling of MNR cultured with and without phytosterols. In addition, the NADH: flavin oxidoreductase and a water-forming NADH oxidase from Lactobacillus brevis, were successfully overexpressed and heterologously expressed in MNR M3 to improve the intracellular ratio of NAD+/NADH. After 96 h of cultivation, the expression of these two enzymes in MNR M3 resulted in the decrease in intracellular NADH level (by 51 and 67%, respectively) and the increase in NAD+/NADH ratio (by 113 and 192%, respectively). Phytosterols bioconversion revealed that the conversion ratio of engineered stains was ultimately improved by 58 and 147%, respectively. The highest AD (D) conversion ratio by MNR M3N2 was 94% in the conversion system with soybean oil as reaction media to promote the solubility of phytosterols.
Conclusions
The ratio of NAD+/NADH is an important factor for the transformation of phytosterols. Expression of NADH: flavin oxidoreductase and water-forming NADH oxidase in MNR improved AD (D) production. Besides the manipulation of key enzyme activities, which included in phytosterols degradation pathways, maintenance the balance of redox also played an important role in promoting steroid biotransformation. The recombinant MNR strain may be useful in industrial production.
Keywords: Phytosterols biotransformation, Proteomic analysis, NADH: flavin oxidoreductase, NADH oxidase, Mycobacterium neoaurum
Background
Metabolic engineering studies generally focused on genetic manipulation (overexpression or disruption) of the genes that encode enzymes involved in a particular pathway. However, the flux of a cofactor-dependent pathway is controlled through the availability of enzyme, as well as the cofactor and ratio of the reduced to the oxidized form of the cofactor. Therefore, cofactor manipulation is a potentially powerful tool for metabolic engineering [1]. Cofactor engineering, a subset of metabolic engineering, is defined as the manipulation of the cofactors in metabolic pathways and optimize dynamic control of the target metabolic flux. It has been successfully applied in Escherichia coli (E. coli) [2, 3], Lactococcus lactis [1], Saccharomyces cerevisiae [4], Klebsiella pneumoniae [5], Serratia marcescens [6], Torulopsis glabrata [7], and Colletotrichum lini [8]. Nicotinamide adenine dinucleotides (NADH and NAD+) is an important cofactor pair that acts in plenty of oxidation–reduction (redox) reactions and regulates various enzyme activities and genetic processes [5–7, 9]. Therefore, the cofactor pair NAD+ and NADH has a critical effect on the maintenance of the intracellular redox balance, which is a basic condition for the growth and metabolism of microorganisms [4]. The NAD+ manipulation system has been successfully used to improve the production of primary metabolites such as ethanol, 1, 2-propanediol [10], acetoin [5, 6, 11], and pyruvate [7]. The most effective way of adjusting the NAD+/NADH ratio is by introducing an NADH or NAD+ regeneration system. Intracellular NADH or NAD+ is easily regenerated in situ by expressing an NAD+-dependent formate dehydrogenase (increase of intracellular NADH availability) or an NADH oxidase (high NAD+/NADH ratio), respectively [6, 12]. Furthermore, the overexpression of the gene of pncB, which encodes NAPRTase, results in increased total NAD+ level and ratio of NAD+/NADH [2].
Steroid medications are used widely in clinical applications and form an important and large category in the pharmaceutical industry. Androst-4-ene-3, 17-dione (AD) and androst-1, 4-diene-3, 17-dione (ADD) are two versatile C19 steroid precursors that are obtained via the nucleus oxidation and the side chain degradation of phytosterols by microorganisms [13, 14]. The process of AD (D) production was carried out using multi-catabolic enzymes promoting many consecutive reactions and not all enzymes included in this complicated process were well studied so far. In general, the manipulation of the key enzyme activities included in phytosterols nucleus oxidation or the side chain cleavage pathways could reportedly enhance the AD (D) production, such as the deletion of 3-ketosteroid-1-dehydrogenase [15, 16], or the overexpression of cholesterol oxidase [17], 3β-hydroxysteroid dehydrogenases [18] and 3-ketosteroid-9α-hydroxylase [15]. However, the overexpression, deletion, or introduction of heterologous genes in target metabolic pathways does not always result in the desired phenotype [19]. Given the multi-catabolic enzyme biotransformation of phytosterols into AD, the cofactors are closely related to phytosterols side-chain degradation. As postulated, the side chain with one mole of sitosterol is selectively removed, as follows: β-sitosterol + 21 H2O + 4 ATP + 7 GDP + 7 Pi + 10 FAD + 21 NAD+ = AD (D) + 21/2 CO2 + 4 AMP + 4 PPi + 7 GTP + 10 FADH2 + 21 NADH + 21 H+ [20, 21]. Maintenance of the redox balance or the regeneration activity of cofactors is supposedly a rate-limiting factor in steroid synthesis. However, given the complication of phytosterols biotransformation pathways, studies on the relationship of cofactors or NAD+/NADH ratio to the AD (D) production are few.
In this work, cofactor manipulation was used in phytosterols biotransformation. First, the connection of phytosterols biotransformation with the intracellular NAD+/NADH ratio in Mycobacterium neoaurum TCCC 11978 (MNR M3) was analyzed by adding nicotinic acid (NA) in the broth. Then, the key enzymes that related to NAD (H) regulation and phytosterols degradation were identified according to the analysis of comparative proteomic of M. neoaurum cultured with and without phytosterols. Afterward, the cofactor engineering was conducted by introduction of NAD+ regeneration system into MNR M3 to increase the availability of NAD+/NADH ratio for AD (D) production based on the proteomic analysis.
Methods
Strain and plasmid construction
The strains, plasmids, and primers used in this study are listed in Table 1.
Table 1.
Bacterial strains, plasmids and primers used in this study
| Strains, plasmids, and primers | Significant properties | Source or purpose |
|---|---|---|
| Strains | ||
| Mycobacterium neoaurum TCCC 11028 M3 (MNR M3) | Wild type | Tianjin University of Science and Technology Culture Collection Center (TCCC) |
| Lactococcus lactis subsp. cremoris NZ9000 | Source of the nox gene | Tianjin University of Science and Technology Culture Collection Center (TCCC) |
| E. coli DH5a | General cloning host | Transgen Biotech |
| MNR M3N1 | MNR M3 containing plasmid pMV261-nox-1 | This work |
| MNR M3N2 | MNR M3 containing plasmid pMV261-nox-2 | This work |
| Plasmids | ||
| pMV261 | Mycobacterial replicating vector carrying the BCG hsp60 promoter, kan | Dr. W. R. Jacobs Jr. (Howard Hughes Medical Institute), for providing plasmid pMV261 |
| pMV261-nox-1 | pMV261, contain nox gene from MNR M3, hsp60, kan, BamHI/HindIII | This work |
| pMV261-nox-2 | pMV261, contain nox gene from Lactococcus lactis subsp. cremoris NZ9000, hsp60, kan, BamHI/salI | This work |
| Primers | ||
| 16 s rRNA-f-RT | ACCAGCGTCCTGTGCATGTC | Quantitative RT-PCR |
| 16 s rRNA-r-RT | AGTACGGCCGCAAGGCTAAAAC | Quantitative RT-PCR |
| Nox-1-f-RT | GGAACAGGTACATGGGGTTG | Quantitative RT-PCR for nox-1 |
| Nox-1-r-RT | GAAGTGGCTGGAAGAAGACG | Quantitative RT-PCR for nox-1 |
| nox-1-f | CGCGGATCCAATGAACACCCAGCCGAAAGT | nox-1 amplification |
| nox-1-r | CCCAAGCTTTCAGACCGTGAGGGTGTCCG | nox-1 amplification |
| nox-2-f | 5′-CGGGGATCCGAAAATCGTAGTTATCGGTA-3′ | nox-2 amplification |
| nox-2-r | 5′-GCGTCGACTTATTTGGCATTCAAAGCTG-3′ | nox-2 amplification |
| PMV-f | 5-TAGGCGAGTGCTAAGAATAACGTTG-3 | Amplification |
Mycobacterium neoaurum TCCC 11978 (MNR M3) obtained from Tianjin University of Science and Technology Culture Collection Center (TCCC), Tianjin, China, was a spontaneous mutant of M. neoaurum TCCC 11028 (MNR) strain. AD accumulated in the broth as a major product by MNR M3. The amount of ADD was too low, less than 5% of the total product in the medium. The AD (D) concentration is the total sum of the two products. MNR M3C2 is the ksdD gene replacement strain MNR M3ΔksdD::ksdD-MNR which was constructed by homologous recombination [16]. The nox-1 gene was amplified through the polymerase chain reaction from the total DNA of MNR M3, using the primers nox-1-f and nox-1-r that generate BamHΙ and HindIIΙ sites. The nox-2 gene was amplified from the total DNA, which was isolated from Lactococcus lactis subsp. cremoris NZ9000 (L. lactis subsp. cremoris NZ9000), using the primers nox-2-f and nox-2-r that generate BamHΙ and SalΙ sites. Mycobacterial replicating vector pMV261 which harbored the Kanamycin (kan) resistance was the common plasmids used in Mycobacterium for gene expression [22]. The amplified fragments were digested by BamHI and HindIII or BamHI and SalI, respectively. Then the treated fragments were inserted into the vector pMV261, which was treated with the same restriction enzymes to generate the vector pMV261-nox-1 and pMV261-nox-2. The pMV261-nox-1 and pMV261-nox-2 vectors were imported into MNR M3 via electroporation. The transformants were screened in a Luria–Bertani medium agar plate containing kan. The selected recombinants were then designated as MNR M3N1 and MNR M3N2 for further characterization.
Chemicals and culture conditions
The phytosterols substrate used is a sterol mixture contained (by weight percentage) 51.7% β-sitosterol, 27.2% stigmasterol, 17.1% campesterol, and 4.0% brassicasterol (COFCO Tech Bioengineering Co., Ltd., Tianjin). Standards AD and ADD were purchased from Sigma-Aldrich Co. (USA). All chemical solvents and salts were of analytical grade or higher. The cultivation and bioconversion of microorganisms and the preparation and analysis of transformation products, were performed following the procedures described by Shen et al. [23]. The minimal medium contained (g/L): glucose 10, MgSO4 0.5, K2HPO4 0.5, (NH4)2HPO4 3.5, citric acid 2, and ammonium iron citrate 0.05 with pH of 7.2. Experiments were conducted under different culture conditions with phytosterols (5 g/L) or soybean oil (16%) and Tween 80 (0.5%) in minimal medium. Different concentrations of NA were added to the medium at the start of fermentation. In the phytosterols-free medium, the growth of cell was measured through optical density. However, the cell growth in the phytosterols-contained culture broth is difficult to measure by using this method. In this study, the cell growth measurement in the medium with phytosterols was conducted following the method by Meyers et al. [24]. The amount of protein was related to the dry cell weight (DCW) obtained using an adequate calibration curve: The growth of cell was represented by DCW. All experiments were performed in triplicate and the data were statistically analyzed by one-way ANOVA.
Determination of NAD+ and NADH concentrations
The intracellular concentrations of NADH and NAD+ were determined as described by Zhang et al. [12].
Assay of NOX activity
0.2 g wet cells of the strains were washed with Tris–HCl buffer (50 mM Tris–HCl, pH 7.5, 0.2 M NaCl, 1% Triton X-100) twice, and resuspended in the same buffer. Cells were then disrupted by sonication at 4 °C for 10 min by a cell sonicator. The homogenate was centrifuged at 12,000 r/min for 30 min at 4 °C. The supernatant was stored in − 80 °C for further research. NOX activity was determined through a photometer assay at 340 nm using 0.1 mM NADH and 0.1 M potassium phosphate buffer at pH 7.0. The reaction was initiated by adding 0.1 mL of cell extracts to the 0.9 mL reaction mixture, and the decrease in absorbance at 340 nm was determined to calculate the NADH concentration. A unit of NOX activity is defined as the amount that catalyzes the oxidation of 1 μmol NADH to NAD+ per minute. Protein concentrations were measured by the method described by Bradford.
Protein analysis and identification
MNR M3C2 was grown in minimal medium. Experiments were conducted under different culture conditions with phytosterols and without phytosterols in minimal medium. The cells were harvested after 60 h cultivation. The collected sediment was washed with phosphate buffer saline at pH 7.2 thrice, sonicated on ice using a high intensity ultrasonic processor (Scientz) in lysis buffer (8 M Urea, 50 mM Tris -HCl, pH 7.5, 1% Nonidet P 40, 1% Sodium deoxycholate, 2 mM Ethylenediaminetetraacetic acid, 5 mM Dithiothreitol, 1% Protease inhibitor), and centrifuged at 20,000g for 10 min at 4 °C. The protein contents in supernatural fluids were estimated using 2D Quant kit. Proteins were prepared and analyzed using tandem mass tag (TMT)—based LC–MS/MS. More specifically, the protein solution was reduced with 5 mM dithiothreitol for 1 h at 37 °C and alkylated with 20 mM iodoacetamide for 45 min at room temperature in darkness. For trypsin digestion, the protein sample was diluted by adding 100 mM triethylamine borane. Finally, trypsin was added at 1:50 trypsin-to-protein mass ratio for the first digestion overnight and 1:100 trypsin-to-protein mass ratio for a second 4 h-digestion. Approximately 300 μg protein for each sample was digested with trypsin for the following experiments. After trypsin digestion, peptide was desalted by Strata X C18 SPE column (Phenomenex) and vacuum-dried. Peptide was reconstituted in 0.5 M triethylamine borane and processed according to the manufacturer’s protocol for 6-plex TMT kit (Pierce). TMT-labeled samples were diluted to 10 mM HCOONH4 buffer (NH3H2O, pH 10) before HPLC on Waters XBridge Shield C18 RP column, 3.5 μm, 4.6 × 250 mm. The flow rate used for reversed-phase column separation is 1 mL/min with mobile phase A (10 mM HCOONH4) and mobile phase B (10 mM HCOONH4, 80% ACN). A solvent gradient system was used: 0–5 min, 2–10% B; 5–55 min, 10–35% B; 55–65 min, 35–90% B; 65–70 min, 90% B; 70–75 min, 90–2% B; 75–80 min, 2% B. In total, 20 fractions were pooled for each sample and dried by vacuum centrifuge. The peptides were separated by a linear gradient formed from 2% ACN, 0.1% FA (mobile phase A), and 80% ACN, 0.1% FA (mobile phase B). A solvent gradient system was used: 0–5 min, 2% B; 5–8 min, 2–11% B; 8–53 min, 11–20% B; 53–55 min, 20–80% B; 55–58 min, 80% B; 58–60 min, 80–2% B; 60–65 min, 2% B. at a flow rate of 300 μl/min. MS analysis was performed on a Thermo Scientific Q Exactive plus. The electrospray voltage applied was 2.0 kV. MS spectra were acquired across the mass range of 350–1500 m/z in high resolution mode using 250 ms accumulation time per spectrum. For data analysis, raw data (.raw) was converted into peak lists (.mgf) by Proteome Discoverer. The database used in searching was uniprot reference proteome M. neoaurum VKM Ac-1815D (Uniprot: UP000018763) [25]. Mass error was set to 20 ppm for precursor ions and 0.02 Da for fragment ions. Carbamidomethylation on Cys was specified as fixed modification and oxidation on methionine was specified as variable modifications. Protein quantification data with relative expressions of > 1.5 and < 0.67 and p values of < 0.01 were selected to ensure the authenticity of up- and down-regulations. Gene ontology (GO) (http://www.geneontology.org/) was used to investigate the potential functions of peptide precursors. The ontology covers three domains, as follows: cellular component, molecular function, and biological process. Kyoto Encyclopedia of Genes and Genomes (KEGG) database (http://www.genome.jp/kegg/) was used to annotate the protein pathway.
Isolation of RNA and quantitative RT-PCR analyses
MNR M3 was grown under different culture conditions with phytosterols and without phytosterols in minimal medium. The cells were harvested after 60 and 72 h cultivation (late logarithmic phase) and preserved at − 80 °C. Total RNA was isolated using Eastep® Super Total RNA Extraction Kit (Promega, Shanghai) according to instructions of supplier. The resulting mixture was treated with DNAse I. For reverse transcription, cDNA synthesis was performed using the PrimeScript™ RT reagent Kit with gDNA Eraser (Perfect Real Time) (Takara) with 0.3 μg of total RNA following the manufacturer’s instructions. Quantitative RT-PCR analyses of cDNA samples were performed on the StepOneTM RealTime PCR System (Applied Biosystems). The above cDNA sample was completely mixed with 0.2 μM specific oligonucleotides, 10 μL of AceQ® qPCR SYBR® Green Master Mix, and 0.4 μL of ROX Reference Dye 1 in 20 μL of reaction mixture. The nucleotide sequences of primers used in this study for target and reference genes are listed in Table 1. Quantitative RT-PCR analyses were performed as follows: 5 min pre-denaturing, 40 cycles of 95 °C for 10 s, 60 °C for 30 s, followed by melting curve stage from 60 to 95 °C. Each gene was measured in triplicate from three independent tests. The cDNA amplification efficiency of samples, internal standards (16S rRNA), and calibrators (samples without induction of phytosterols) were equivalently modulated to ensure that the relative amount of mRNA could be processed using the 2−ΔΔCt algorithm [26]. Each gene was measured in triplicate from three independent tests.
Results
Interaction between phytosterols degradation process and the intracellular NAD+/NADH ratio
To better understand the connection of the intracellular NAD (H) with AD (D) production in MNR M3, the intracellular NAD+/NADH ratio and AD (D) production during biotransformation were examined at regular intervals; on the other hand, NA (vitamin precursor of NAD+) was added to the fermentation system to change the intracellular redox status.
As shown in Fig. 1a, the ratio of NAD+/NADH constantly changed during the fermentation. This outcome is consistent with the studies of Ji et al. [5] and Wu et al. [8]. During the stage from 48 to 96 h, the NAD+/NADH ratio in MNR M3 increased. Following, the NAD+/NADH ratio decreased quickly after 96 h and the highest NAD+/NADH ratio was obtained at 96 h. The dynamics of the accumulation of AD (D) was also studied. From 48 to 72 h, the productivity of AD (D) is 7.5 mg/(L h), which increased to 10.4 mg/(L h) at the next stage (72–96 h). After 96 h, the rate remarkably decreased to 2.9 mg/(L h). The productivity of AD (D) showed a similar trend with the change of NAD+/NADH ratio. Thus, we preliminarily inferred a correlation between the NAD+/NADH ratio and the AD (D) productivity during the late biotransformation period (after 96 h) in MNR M3.
Fig. 1.
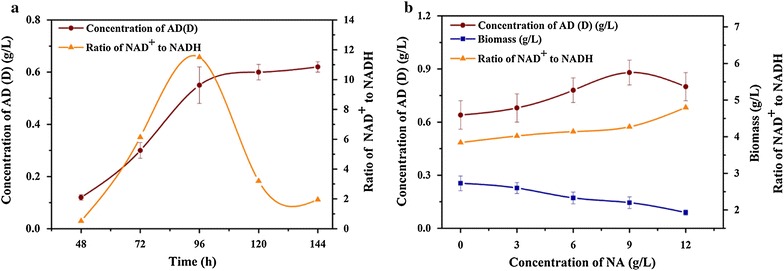
a Time courses of the AD (D) production and NAD+/NADH ratio during phytosterols transformation with MNR M3. b Effect of NA on cell growth, NAD+/NADH ratio, and AD (D) production during phytosterols biotransformation with MNR M3. Biotransformation conditions: 50 mL fermentation medium in 250 mL shake flask, 5 g/L phytosterols, 30 °C, 140 r/min for 120 h transformation. The error bars represent mean ± SD (n = 3)
NA is used to elevate the intracellular NAD+ level through the salvage pathway in M. tuberculosis [27]. A suitable concentration of NA in the medium is important for the efficient production [2, 28]. Thus, a series of NA concentrations with phytosterols was simultaneously added to the fermentation medium of MNR M3. From Fig. 1b, the AD (D) production was continuously increased with increasing NA concentration (0–9 g/L), and the highest level of AD (D) (0.88 g/L) was achieved with 9 g/L NA. This value was 37.5% higher than in the control which without NA (0.64 g/L). The addition of NA could increase the NAD+/NADH ratio during the late biotransformation period as well. When 12 g/L NA was added into the fermentation system, the level of NAD+/NADH ratio was 25% higher than that of the control (without NA). However, the weak growth was observed with increasing NA concentration in the fermentation medium, which is in accordance with that in Colletotrichum lini [8]. The significantly inhibition of the cell growth at high concentration of NA might lead to the AD (D) production with 12 g/L NA was lower than that with 9 g/L NA. As stated above, high levels of NAD+/NADH ratio was obtained and the production of AD (D) was enhanced with the addition of NA. We could get that the regeneration activity of NAD+ had a positive effect on the production of AD (D).
Selection of the gene targets for NAD+ and NADH modification to enhance the AD (D) production
It’s necessary to establish an efficient way to generate NAD+ cofactor by genetic modification in MNR M3. In order to investigate whether the proteins regulate the redox balance of cofactor and bring a positive influence on phytosterols metabolic pathway, we detected the changes in proteome of MNR M3C2 cultured with and without phytosterols in minimal medium.
A total of 19025 peptides and 3727 proteins were identified, among which 299 proteins showed significant changes, as follows: 174 proteins were up-regulated and 125 proteins were down-regulated. The changed proteins included in cofactor metabolism were analyzed. The accession number, protein name, and fold change (with phytosterols/without phytosterols) for proteins were available in Table 2. The regeneration of NAD+ from NADH was mainly through electron transfer chain (ETC), oxygen was used as the final electron acceptor and ATP was produced [7]. So the proteins involved in ETC were analyzed, the NADH dehydrogenase (NuoL, NuoG, NuoC, and NuoE) were down-regulated obviously due to the existence of phytosterols. This finding is consistent with the study stating that sterol substrates and products such as ADD and AD inhibit the cell growth and impair the cell’s respiratory chain [29, 30]. Apart from the ETC pathway, expression of an NADH: flavin oxidoreductase (NOX-1), which can oxidize NADH using O2 to produce NAD+ and H2O, was also detected in MNR M3C2. It was noteworthy that the expression of this protein was significantly up-regulated by 3.1-fold with phytosterols in minimal medium. By analyzing the proteome of MNR M3 with and without phytosterols, we speculated that the up-regulated protein NOX-1 performed important functions in phytosterols metabolism and cofactor regulation pathways.
Table 2.
Identified differentially expressed proteins of MNR M3C2 in the presence of phytosterols
| Protein accession | Protein description | Changed ratio |
|---|---|---|
| V5X8K9 | NADH-quinone oxidoreductase subunit L | 0.497 |
| V5X8L5 | NADH-quinone oxidoreductase subunit G | 0.561 |
| V5XBK6 | NADH-quinone oxidoreductase subunit C | 0.501 |
| V5X9W2 | NADH-quinone oxidoreductase subunit E | 0.479 |
| V5X9K2 | NADH:flavin Oxidoreductase | 3.115 |
Changed ratio presents the ratio of proteins with phytosterols in the fermentation medium to that without phytosterols
To validate the results from proteomic analysis, the transcription level of NOX-1 in MNR M3 with and without phytosterols was assayed by quantitative RT-PCR. As shown in Fig. 2, when compared with that of MNR M3 cultured without phytosterols, the relative transcription of NOX-1 increased by 4.56 and 2.89 times after cultivation for 60 and 72 h, respectively. This finding indicated the credibility of the up-regulated ratio of NOX-1 with phytosterols showed in proteomic analysis.
Fig. 2.

Transcription levels of NADH: flavin Oxidoreductase in MNR M3 induced by phytosterols
Expression of NOX in MNR M3 and its effect on NAD+/NADH ratio
Both NOX-1 and NADH oxidase (NOX-2) catalyze the oxidation of NADH to NAD+ by using molecular oxygen as the electron acceptor [31, 32]. In order to improve the NAD+/NADH ratio for phytosterols biotransformation, NOX-1 was overexpressed in MNR M3. Moreover, the NOX-2 from L. lactis subsp. cremoris NZ9000 was also chosen to express in MNR M3 as it can lead to a dramatically increased NAD+/NADH ratio [33, 34]. As stated in the Materials and Methods Section, the confirmed recombinants MNR M3N1 and MNR M3N2 were obtained.
The specific activity of NOX and the content of NAD+ and NADH were measured when the cells entered the end stage of exponential phase. As shown in Table 3, the specific activity of NADH oxidase (0.86 U/mg protein) in the recombinant strain MNR M3N2 was higher than that of MNR M3N1 (0.32 U/mg protein), whereas the NADH oxidase activity in the parent strain was not detected. This finding indicated that NOX was actively produced in the recombinant strains, and the expression of NOX-2 in MNR M3N2 had a higher catalytic activity.
Table 3.
Intracellular NADH oxidase activities, NAD (H) concentrations and ratio of NAD+ to NADH in MNR M3, MNR M3N1 and MNR M3N2
| Strains | MNR M3 | MNR M3N1 | MNR M3N2 |
|---|---|---|---|
| NADH oxidase activity (U/mg) | ND | 0.32 ± 0.02 | 0.86 ± 0.02 |
| NAD+ (μmol/g DCW) | 9.34 ± 0.50 | 9.71 ± 0.33 | 9.11 ± 0.72 |
| NADH (μmol/g DCW) | 0.90 ± 0.15 | 0.44 ± 0.05 | 0.30 ± 0.02 |
| Ratio of NAD+ to NADH | 10.4 | 22.1 | 30.4 |
Biotransformation conditions: 50 mL fermentation medium in 250 mL shake flask, 30 °C, 140 r/min, 96 h. All experiments were performed in triplicate
The effect of NOX expression on cell growth was studied during aerobic cultivations of strains MNR M3, MNR M3N1, and MNR M3N2. As shown in Fig. 3a, cell growth trends of these strains shared typical curves that could be divided into four stages, as follows. They grew slowly at the first 36 h. Then, they grew fast (36–84 h). The growth rates were lowered down (84–96 h) and cell concentrations were unchanged after 96 h of cultivations. However, the growth rate of the NOX expression strains were slower than that of the parent strain, the growth rate of MNR M3N1 was better than that of MNR M3N2 with higher NOX expression. The NOX expression had a negative effect on cell growth.
Fig. 3.
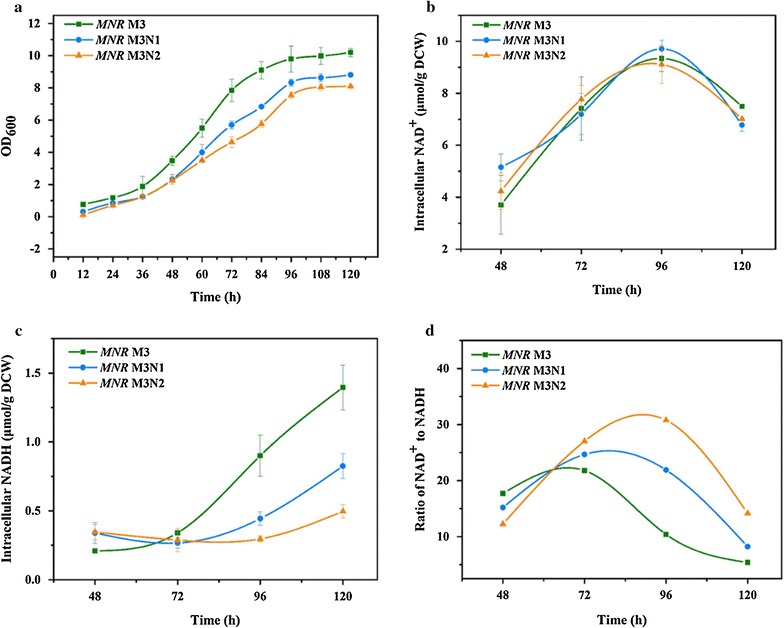
Time courses of various parameters during cultured in minimal medium with MNR M3, MNR M3N1, MNR M3N2, respectively. It shows the cell growth (a), the concentrations of intracellular NADH (b), NAD+ (c), and NAD+/NADH ratio (d). Biotransformation conditions: 50 mL fermentation medium in 250 mL shake flask, 30 °C, 140 r/min for 120 h culture. The error bars represent mean ± SD (n = 3)
The expression of NOX in MNR M3 was expected to increase the overall intracellular NAD+ pool and NAD+/NADH ratio, thereby improving the flux of NAD+-dependent pathways. As shown in Fig. 3b, c, intracellular concentrations of NADH and NAD+ continuously changed during various stages of the cell growth process among the parent and the engineered strains. In the three strains, levels of NAD+ were increased during growth phases and when cells entered non-growth phase, the NAD+ levels in these three strains were decreased. No remarkable difference on the NAD+ level among these three strains was observed. However, levels of NADH in the three strains were different. The NADH levels remain unchanged at the first 72 h, and when cells entered the end stage of exponential and the non-growth phase, the NADH levels in these three strains were increased. The increased rate of NADH level in the engineered strains was lower than that in the parent strain, and MNR M3N2 with the higher NOX expression has the lowest increase of NADH level among these three strains. NOX expression led to a decrease of NADH pools comparing with the parent strain. Meanwhile, the ratio of NAD+/NADH ratio among these three strains was studied (Fig. 3d). The production of NOX in MNR M3 led to the increase of NAD+/NADH ratio. After 96 h, the ratio in MNR M3N1 and MNR M3N2 was 113 and 192% higher than that in the parent strain, respectively. This suggests that the redox balance in MNR M3 producing NOX was disturbed, which also explains why cell growth of MNR M3N1 and MNR M3N2 was affected (Fig. 3a).
Effect of NOX expression on AD (D) production in the fermentation
The recombinant strains both could produce NOX and disturb the redox balance of cofactor (Table 3, Fig. 3), the NAD+-dependent pathways were expected to be enhanced in the recombinant strains. To characterize the phytosterols metabolic process of MNR M3 in response to the introduction of the NOX: NAD+ regeneration system, conversion ratio of major metabolite of AD (D) in MNR M3, MNR M3N1, MNR M3N2 were determined. The bioavailability of steroid hormone in the bioconversion process is low because of the remarkably high hydrophobic nature of steroids. Furthermore, most of the enzymes involved in phytosterols degradation are promoted by oxygen. To improve the production of steroid products in aqueous systems, natural oils have been widely introduced to microbial transformations of steroids [35], as it could increase substrate solubility and improve the dissolved oxygen in steroid biotransformation [21, 36]. So soybean oil was employed here to evaluate the productivity of AD (D) in the constructed strains. As shown in Fig. 4, the time courses of conversion ratio and phytosterols consumption of MNR M3, MNR M3N1, and MNR M3N2 were studied. The highly expressed NOX-2 in MNR M3N2 increased the conversion ratio (94%) when compared with that in the parent strain (38%), and the strain with moderate expression of NOX-1 (MNR M3N1) increased the conversion ratio (60%) as well. When compared with MNR M3, the AD (D) conversion ratio in MNR M3N1 and MNR M3N2 increased by 58 and 147%, respectively. Moreover, the productivity of AD (D) were analyzed in the phytosterols conversion to AD (D). Among these three strains, MNR M3N2 with the highest NOX production showed the highest productivity at every fermentation stage. Moreover, all the substrate of phytosterols (5 g/L) was run out in MNR M3N2 fermentation at 144 h. This indicated that the fluxes towards oxidative and reductive metabolism of phytosterols increased upon introduction of NAD+ regeneration system in MNR M3. Hence, the increase of the NAD+/NADH ratio induced by the expression of NOX is important for AD (D) production.
Fig. 4.
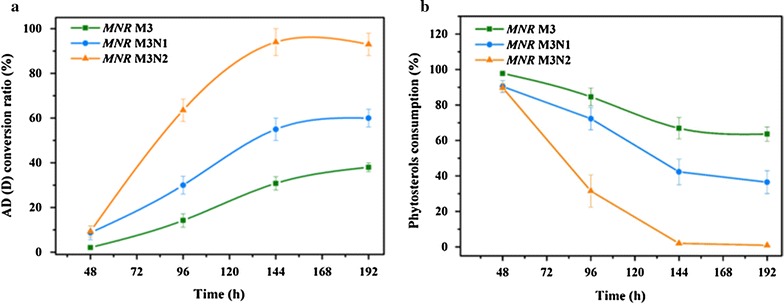
Time courses of phytosterols conversion to AD (D) by MNR M3, MNR M3N1, and MNR M3N2. a conversion ratio. b Consumption of phytosterols. Tests were conducted with 5 g/L phytosterols, 16% soybean oil and 0.5% tween 80 contained in minimal medium. The error bars represent mean ± SD (n = 3)
Discussion
AD and ADD are important steroid medicine intermediates. They are generally obtained via the side chain degradation of phytosterols by microorganisms. The analysis of metabolic pathways associated with AD production from phytosterols showed that either cholesterol oxidase or 3β-hydroxysteroid dehydrogenase is responsible for the first step of the cholesterol degradative pathway, which uses NAD+ as a cofactor and oxidizes 3β-hydroxysterols to 3-ketosteroids [37]. Further cleavage of the alkyl sterol side chain at C17 resulted from the fatty acid β-oxidation [20], and the oxidized cofactor is vital for the pathway. Based on the theoretical stoichiometry in AD (D) biosynthesis pathway, one molecule of β-sitosterol is consumed, and 21 molecules of NADH are generated [20, 21]. The high NAD+ to NADH ratio could be a promoting factor for the AD (D) conversion ratio from phytosterols through the side chain cleavage. As shown in Fig. 1, both the NAD+/NADH ratio and the AD (D) production were increased with the increasing of NA concentration (0–9 g/L). The result was similar to that obtained in the Torulopsis glabrata [7], in which the intracellular NAD+ level and NAD+/NADH ratio increased in the presence of NA.
Cofactor engineering has been widely used to promote the production of important primary metabolites, such as 1, 2-propanediol [10], acetoin [5, 6, 11], and pyruvate [7]. However, the phytosterols degradation process is different from the metabolism of primary metabolites, and is carried out using multi-catabolic enzymes that promote multiple consecutive reactions. Furthermore, the degradation process is closely related to the glucose and fatty acid related metabolic pathways [37, 38]. The complexity of the phytosterols degradation process leads to the limited use of cofactor engineering in AD (D) production. Therefore, powerful technological approaches based on proteomics were used in this study. NADH: flavin oxidoreductase in MNR M3 was identified and supposed to the key enzyme to regulate the NAD+/NADH balance for AD (D) production through the analysis of comparative proteomic of MNR cultured with and without phytosterols (Table 2). The overexpression of NOXs (NOX-1 and NOX-2) in MNR M3 was a useful tool for strengthening the phytosterols metabolism and for studying the interaction between the NAD+ level and metabolic fluxes. As shown in Figs. 3 and 4, the higher activity of NADH oxidase, the higher NAD+/NADH ratio and AD (D) production were got in MNR M3N2.
The large increase of the NAD+/NADH ratio due to the introduction of the NAD+ regeneration system triggered a dramatic variation of phytosterols metabolism. As shown in Fig. 5, the process from phytosterols to AD that takes NAD+, with NADH, propionyl-CoA, and acetyl-CoA, is generated [37, 38]. Moreover, propionyl-CoA and acetyl-CoA are degraded mainly by the tricarboxylic acid (TCA) pathway. So, a sufficient supply of NAD+ was essential to the phytosterols degradation pathway [20, 21]. By means of the proteomic analysis, the ETC pathway and NOX-1 with oxygen as the electron acceptor was found important for the regeneration of NAD+ from NADH in MNR (Table 2). However, NADH-quinone oxidoreductase which was the key enzyme included in ETC pathway, was inhibited, and this finding was consistent with the results of a previous study in which sterol substrates and products (such as ADD and AD) impair the cell’s respiratory chain [29, 30]. Thus, the NOX played an important role in NAD+ regeneration for AD (D) production. Finally, the enhancement of intracellular NAD+ regeneration system resulted in the strengthening of phytosterols degradation and improvement of AD (D) production and productivity in aerobic fermentation.
Fig. 5.

Biotransformation pathways from phytosterols to AD (D) by MNR [20, 36, 37]. According to the protein analysis of MNR, the red line represents the up-regulated pathway of MNR cultured with phytosterols; the green line represents the down-regulated pathway of MNR cultured with phytosterols
Conclusions
Increasing the intracellular NAD+/NADH ratio could be beneficial to the production of AD (D) and that the introduction of NAD+ regeneration system into MNR is a powerful engineering tool to enhance the metabolic flux for the desired metabolites. The successful expression of NOXs in MNR M3 resulted in a large increase in NAD+/NADH ratio, thereby markedly enhancing the phytosterols metabolism pathways. The obtained data proved that, besides the manipulation of key enzyme activities, which are included in phytosterols degradation pathways, the maintenance of redox balance and the regeneration activity of cofactors also played an important role in promotion the steroid biotransformation. This study provides a useful strategy for enhancing the accumulation of NAD+-dependent microbial metabolites in the microbial biotransformation of steroids.
Authors’ contributions
LS performed most of the experiments, data analyses, and interpretation. YS designed and supervised the research and edited the manuscript aspects of this work. WZ designed and participated in the experimental aspects of this work. TG helped in the design and participated in the experimental aspect. ZS helped edit the manuscript. MW supervised the research and edited the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data generated or analyzed in this study are included in the published article.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This article does not contain any studies involving human or animal participants.
Funding
This work was supported by the National Natural Science Foundation of China (21276196 and 21406167); Key Project of Chinese Ministry of Education (213004A); and Tianjin Programs for Science and Technology Development (15ZCZDSY00510).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- NAD (H)
nicotinamide adenine dinucleotides
- AD
androst-4-ene-3, 17-dione
- ADD
androst-1, 4-diene-3, 17-dione
- NOX-1
NADH: flavin oxidoreductase
- NOX-2
NADH oxidase
- MNR M3
Mycobacterium neoaurum TCCC 11978
- NA
nicotinic acid
- DCW
dry cell weight
- kan
Kanamycin
- TMT
tandem mass tag
- FDR
false discovery rate
- GO
gene ontology
- KEGG
Kyoto encyclopedia of genes and genomes
- ETC
electron transfer chain
- TCA
tricarboxylic acid cycle
Contributor Information
Liqiu Su, Email: suliqiu123456@163.com.
Yanbing Shen, Phone: +86 22 60601256, Email: shenyb@tust.edu.cn.
Wenkai Zhang, Email: 1255652028@qq.com.
Tian Gao, Email: 2846328699@qq.com.
Zhihua Shang, Email: 1017442764@qq.com.
Min Wang, Phone: +86 22 60601256, Email: minw@tust.edu.cn.
References
- 1.De Felipe FL, Kleerebezem M, de Vos WM, Hugenholtz J. Cofactor engineering: a novel approach to metabolic engineering in Lactococcus lactis by controlled expression of NADH oxidase. J Bacteriol. 1998;180:3804–3808. doi: 10.1128/jb.180.15.3804-3808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berrios-Rivera SJ, San KY, Bennett GN. The effect of NAPRTase overexpression on the total levels of NAD, the NADH/NAD+ ratio, and the distribution of metabolites in Escherichia coli. Metab Eng. 2002;4:238–247. doi: 10.1006/mben.2002.0229. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez AM, Bennett GN, San KY. Effect of different levels of NADH availability on metabolic fluxes of Escherichia coli chemostat cultures in defined medium. J Biotechnol. 2005;117:395–405. doi: 10.1016/j.jbiotec.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Heux S, Cachon R, Dequin S. Cofactor engineering in Saccharomyces cerevisiae: expression of a H2O-forming NADH oxidase and impact on redox metabolism. Metab Eng. 2006;8:303–314. doi: 10.1016/j.ymben.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Ji XJ, Xia ZF, Fu NH, Nie ZK, Shen MQ, Tian QQ, He H. Cofactor engineering through heterologous expression of an NADH oxidase and its impact on metabolic flux redistribution in Klebsiella pneumonia. Biotechnol Biofuels. 2013;6:7–15. doi: 10.1186/1754-6834-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun JA, Zhang LY, Rao B, Shen YL, Wei DZ. Enhanced acetoin production by Serratia marcescens H32 with expression of a water-forming NADH oxidase. Bioresource Technol. 2012;119:94–98. doi: 10.1016/j.biortech.2012.05.108. [DOI] [PubMed] [Google Scholar]
- 7.Liu LM, Li Y, Shi ZP, Du GC, Chen J. Enhancement of pyruvate productivity in Torulopsis glabrata: increase of NAD+ availability. J Biotechnol. 2006;126:173–185. doi: 10.1016/j.jbiotec.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y, Li H, Zhang XM, Gong JS, Li H, Rao ZM, Shi JS, Xu ZH. Improvement of NADPH-dependent P450-mediated biotransformation of 7a, 15a-diOH-DHEA from DHEA by a dual cosubstrate-coupled system. Steroids. 2015;5:6–11. doi: 10.1016/j.steroids.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Foster JW, Park YK, Penfound T, Fenger T, Spector MP. Regulation of NAD metabolism in Salmonella typhimurium: molecular sequence analysis of the bifunctional nadR regulator and the nadA-pnuC operon. J Bacteriol. 1990;172:4187–4196. doi: 10.1128/jb.172.8.4187-4196.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berrios-Rivera SJ, San KY, Bennett GN. The effect of carbon sources and lactate dehydrogenase deletion on 1, 2-propanediol production in E. coli. J Ind Microbiol Biotechnol. 2003;30:34–40. doi: 10.1007/s10295-002-0006-0. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Zhang RZ, Bao T, Rao ZM, Yang TW, Xu MJ, Xu ZH, Li HZ, Yang ST. The rebalanced pathway significantly enhances acetoin production by disruption of acetoin reductase gene and moderate-expression of a new water-forming NADH oxidase in Bacillus subtilis. Metab Eng. 2014;23:34–41. doi: 10.1016/j.ymben.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Zhang YP, Huang ZH, Du CY, Li Y, Cao ZA. Introduction of an NADH regeneration system into Klebsiella oxytoca leads to an enhanced oxidative and reductive metabolism of glycerol. Metab Eng. 2009;11:101–106. doi: 10.1016/j.ymben.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Egorova OV, Gulevskaya SA, Puntus IF, Filonov AE, Donova MV. Production of androstenedione using mutants of Mycobacterium sp. J Chem Technol Biotechnol. 2002;77:141–147. doi: 10.1002/jctb.536. [DOI] [Google Scholar]
- 14.Malaviya A, Gomes J. Enhanced biotransformation of sitosterol to androstenedione by Mycobacterium sp. using cell wall permeabilizing antibiotics. J Ind Microbiol Biotechnol. 2008;35:1235–1239. doi: 10.1007/s10295-008-0419-5. [DOI] [PubMed] [Google Scholar]
- 15.Yao K, Xu LQ, Wang FQ, Wei DZ. Characterization and engineering of 3-ketosteroid-delta-1-dehydrogenase and 3-ketosteroid-9α-hydroxylase in Mycobacterium neoaurum ATCC 25795 to produce 9α-hydroxy-4-androstene-3, 17-dione through the catabolism of sterols. Metab Eng. 2014;24:181–191. doi: 10.1016/j.ymben.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Xie RL, Shen YB, Qin N, Wang YB, Su LQ, Wang M. Genetic differences in ksdD influence on the ADD/AD ratio of Mycobacterium neoaurum. J Ind Microbiol Biotechnol. 2015;42:507–513. doi: 10.1007/s10295-014-1577-2. [DOI] [PubMed] [Google Scholar]
- 17.Yao K, Wang FQ, Zhang HC, Wei DZ. Identification and engineering of cholesterol oxidases involved in the initial step of sterols catabolism in Mycobacterium neoaurum. Metab Eng. 2013;15:75–87. doi: 10.1016/j.ymben.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Uhía I, Galán B, Morales V, García JL. Initial step in the catabolism of cholesterol by Mycobacterium smegmatis mc2 155. Environ Microbiol. 2011;13(4):943. doi: 10.1111/j.1462-2920.2010.02398.x. [DOI] [PubMed] [Google Scholar]
- 19.Wei W, Wang FQ, Fan SY, Wei DZ. Inactivation and augmentation of the primary 3-ketosteroid-delta-1-dehydrogenase in Mycobacterium neoaurum NwIB-01: biotransformation of soybean phytosterols to 4-androstene-3,17-dione or 1,4-androstadiene-3,17-dione. Appl Environ Microb. 2010;76(13):4578–4582. doi: 10.1128/AEM.00448-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szentirmai A. Microbial physiology of side chain degradation of sterols. J Ind Microbiol Biotechnol. 1990;6(2):101–115. [Google Scholar]
- 21.Su LQ, Shen YB, Gao T, Luo JM, Wang M. Improvement of AD biosynthesis response to enhanced oxygen transfer by oxygen vectors in Mycobacterium neoaurum TCCC 11979. Appl Biochem Biotechnol. 2017 doi: 10.1007/s12010-017-2418-3. [DOI] [PubMed] [Google Scholar]
- 22.Wei W, Fan SY, Wang FQ, Wei DZ. Accumulation of androstadiene-dione by overexpression of heterologous 3-ketosteroid D1-dehydrogenase in Mycobacterium neoaurum NwIB-01. World J Microbiol Biotechnol. 2014;30:1947–1954. doi: 10.1007/s11274-014-1614-3. [DOI] [PubMed] [Google Scholar]
- 23.Shen YB, Wang M, Li HN, Wang YB, Luo JM. Influence of hydroxypropyl-β cyclodextrin on phytosterol biotransformation by different strains of Mycobacterium neoaurum. J Ind Microbiol Biotechnol. 2012;39(9):1253–1259. doi: 10.1007/s10295-012-1130-0. [DOI] [PubMed] [Google Scholar]
- 24.Meyers PR, Bourn WR, Steyn LM, Helden PDV, Beyers AD, Brown GD. Novel method for rapid measurement of growth of Mycobacteria in detergent-free media. J Clinical Microbiol. 1998;36(9):2752–2754. doi: 10.1128/jcm.36.9.2752-2754.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shtratnikova VY, Bragin EY, Dovbnya DV, Pekov YA, Schelkunov MI, Strizhov N, Ivashina TV, Ashapkin VV, Donova MV. Complete genome sequence of sterol-transforming Mycobacterium neoaurum Strain VKM Ac-1815D. Genome Announc. 2014;2(1):e01177. doi: 10.1128/genomeA.01177-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Boshoff HI, Xu X, Tahlan K, Dowd CS, Pethe K, Camacho LR, Park TH, Yun CS, Schnappinger D, Ehrt S, Williams KJ, Barry CE. Biosynthesis and recycling of nicotinamide cofactors in Mycobacterium tuberculosis. J Biol Chem. 2008;283(28):19329–19341. doi: 10.1074/jbc.M800694200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang LY, Liu RM, Wang GM, Gou DM, Ma JF, Chen KQ, Jiang M, Wei P, Ouyang PK. Regulation of NAD(H) pool and NADH/NAD+ ratio by overexpression of nicotinic acid phosphoribosyltransferase for succinic acid production in Escherichia coli NZN111. Enzyme Microb Technol. 2012;51:286–293. doi: 10.1016/j.enzmictec.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Malaviya A, Gomes J. Androstenedione production by biotransformation of phytosterols. Bioresource Technol. 2008;99:6725–6737. doi: 10.1016/j.biortech.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 30.Donova MV. Transformation of steroids by actinobacteria: a review. Appl Biochem Microbiol. 2007;43(1):5. doi: 10.1134/S0003683807010012. [DOI] [PubMed] [Google Scholar]
- 31.Baron SF, Hylemon PB. Expression of the bile acid-inducible NADH: flavin oxidoreductase gene of Eubacterium sp. VPI 12708 in Escherichia coli. Biochim Biophys Acta. 1995;1249(2):145–154. doi: 10.1016/0167-4838(95)00034-R. [DOI] [PubMed] [Google Scholar]
- 32.Liu JJ, Yin YP, Song ZY, Li Y, Jiang SS, Shao CW, Wang ZK. NADH: flavin oxidoreductase/NADH oxidase and ROS regulate microsclerotium development in Nomuraea rileyi. World J Microbiol Biotechnol. 2014;30:1927–1935. doi: 10.1007/s11274-014-1610-7. [DOI] [PubMed] [Google Scholar]
- 33.Xu S, Zhou JW, Qin Y, Liu LM, Chen J. Water-forming NADH oxidase protects Torulopsis glabrata against hyperosmotic stress. Yeast. 2010;27:207–216. doi: 10.1002/yea.1745. [DOI] [PubMed] [Google Scholar]
- 34.Vemuri GN, Eiteman MA, McEwen JE, Olsson L, Nielsen J. Increasing NADH oxidation reduces overflow metabolism in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2007;104:2402–2407. doi: 10.1073/pnas.0607469104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gulla V, Banerjee T, Patil S. Bioconversion of soysterols to androstenedione by Mycobacterium fortuitum subsp. fortuitum NcIM 5239, a mutant derived from total sterol degrader strain. J Chem Tech Biotechnol. 2010;85:1135–1141. doi: 10.1002/jctb.2410. [DOI] [Google Scholar]
- 36.Marques MPC, Carvalho F, Carvalho CCCRD, Cabral JMS, Fernandes P. Steroid bioconversion: towards green processes. Food Bioprod Process. 2010;88(1):12–20. doi: 10.1016/j.fbp.2010.01.009. [DOI] [Google Scholar]
- 37.García JL, Uhía I, Galán B. Catabolism and biotechnological applications of cholesterol degrading bacteria. Microb Biotechnol. 2012;5(6):679–699. doi: 10.1111/j.1751-7915.2012.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griffin JE, Gawronski JD, Dejesus MA, Ioerger TR, Akerley BJ, Sassetti CM. High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog. 2011;7(9):e1002251. doi: 10.1371/journal.ppat.1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed in this study are included in the published article.


