Fig. 1.
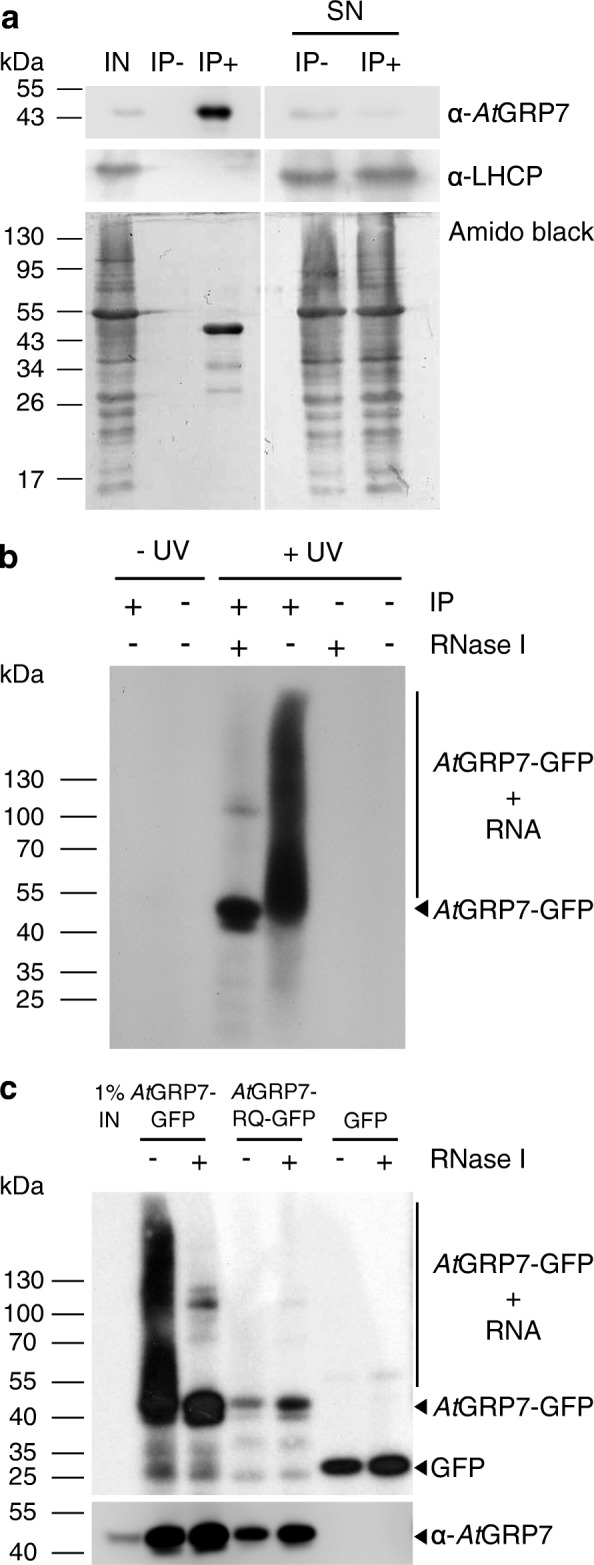
Immunoprecipitation of AtGRP7 protein–RNA complexes from UV crosslinked AtGRP7::AtGRP7-GFP grp7-1 plants. RNA–protein interactions were stabilized by UV irradiation of 16-day-old plants with UV light (254 nm) at 500 mJ/cm2. Lysates were subjected to immunoprecipitation with GFP Trap beads (IP+) and mock precipitation with RFP Trap beads (IP−). a Aliquots of the lysate (input, IN), IP+, IP− and the supernatant (SN) of the precipitations were analyzed by immunoblotting with the α-AtGRP7 antibody. The α-LHCP antibody served as control. For comparison, the membrane was stained with amidoblack. Positions of the molecular weight markers are indicated. b Autoradiogram of RNA–protein complexes from AtGRP7::AtGRP7-GFP grp7-1 plants after UV XL and without UV XL and after precipitation (IP+) or mock precipitation (IP−). Treatment of the precipitate with RNase I (+ RNase) indicates the size of the precipitated protein. c Autoradiogram of RNA–protein complexes of UV crosslinked AtGRP7::AtGRP7-GFP grp7-1 plants, AtGRP7::AtGRP7 R 49 Q-GFP, and AtGRP7::GFP-only plants. Immunoblot against AtGRP7 identifies the precipitated protein (bottom). Marker positions and the location of the AtGRP7-GFP RNA adducts are indicated
