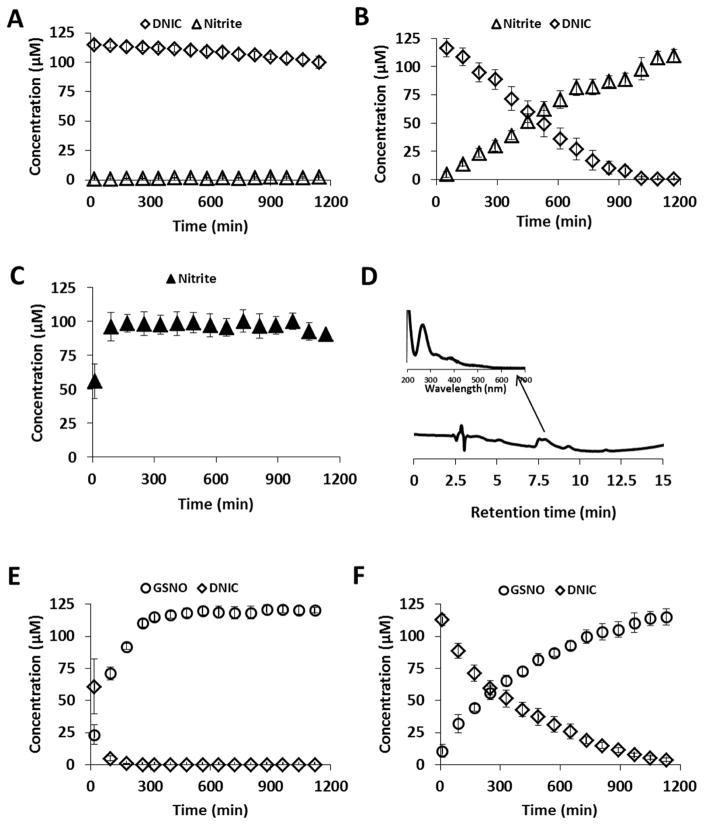
Figure 2. Decomposition of DNIC at neutral and at acidic pH.
DNIC was synthesized from 100 μM of FeSO4, and 100 μm of Proli/NO with GSH in 15 mM Hepes pH 7.4, and analyzed with reverse phase HPLC/UV-Vis detector. Fe:thiol=1:2 (A, C, E), and 1:20 (B, F). (A, B): DNIC decay, and accumulation of nitrite at pH 7.4. (C): Nitrite accumulation in the presence of HgCl2. (D): HPLC traces of the first sample of (C). Spectrum of intermediate species is indicated with arrow at tret=7.7 min. (E, F): DNIC decay, and accumulation of GSNO in the presence of 10% 2N HCl. Diamonds: DNIC, triangles: nitrite, circles: GSNO. Closed triangles: DNIC was supplemented with HgCl2. Data are reported as mean±standard error, N=3.