Abstract
Objectives
Irritable bowel syndrome (IBS) clusters in families, but the familial risk of IBS has not been determined in adoptees. Studying adoptees and their biological and adoptive parents is a strong study design for separating genetic from environmental causes of familial clustering. This nationwide study aimed to separate the biological (genetic) and familial environmental contribution to the familial transmission of IBS.
Methods
We performed a family study for Swedish-born adoptees born from 1951 until 1995, and their biological and adoptive parents. The Swedish Multigeneration Register was linked to the Hospital Register (inpatients and outpatients) for the period 1964–2012 and the Swedish Outpatient Care Register for 2001–2012, and the Swedish Primary Healthcare register for 1989–2012. ORs for IBS were calculated for adoptees with an affected biological parent with IBS compared with adoptees without a biological parent with IBS. The OR for IBS was also determined in adoptees with an adoptive parent with IBS compared with adoptees without an adoptive parent with IBS. Heritability h 2 (±SE) was also determined.
Results
The ORs for IBS were 1.67 in adoptees (95% CI 1.06 to 2.62) of biological parents diagnosed with IBS. The ORs for IBS were 0.88 in adoptees (95% CI 0.48 to 1.63) of adoptive parents diagnosed with IBS. The heritability was 19.5%±8.5%.
Conclusions
The present study indicates that biological (genetic) factors are important for the familial clustering of IBS. The heritability calculated is in the range from twin studies and suggests that heritability may be estimated in adoptees.
Keywords: irritable bowel syndrome, epidemiology, genetics
Summary box.
What is already known about this subject?
Irritable bowel syndrome (IBS) is known to aggregate in families.
Familial aggregation may be due to genetic or environmental factors.
What are the new findings?
IBS is transmitted to adoptees from their biological parents but not to a major degree from their adoptive parents.
The present study suggests that biological (genetic) factors are important in the familial aggregation of IBS among adoptees.
How might it impact on clinical practice in the foreseeable future?
History of IBS in a biological parent is a risk factor for IBS in adoptees.
Genetic studies in order to identify IBS-associated genetic variants might be worthwhile.
Introduction
Irritable bowel syndrome (IBS) is a common chronic functional bowel disorder characterised by abdominal pain or discomfort.1 2 IBS is believed to be a complex disorder or trait,3 that is, any phenotype that does not show classic Mendelian recessive or dominant inheritance due to a single gene locus.4 IBS clusters in families and5–9 familial ORs for IBS among first relatives has been reported to range between 1.75 and 3.1.6–9 The reason for this may be due to shared genes or shared family environmental exposures.10 11 Twin and adoptee studies can help to disentangle genetic and environmental influences.11 Twin studies support the concept that IBS has both genetic and environmental contributions.12–17 The heritability, that is, the fraction of the phenotype variability that can be attributed to genetic variation, has been determined to be 56.9% for functional gastrointestinal disorder in general and between 19% and 48% for IBS in twin studies.12–19 Furthermore, extended family studies may also support a genetic cause of familial clustering.9 While family studies suggest a genetic contribution, recent genetic studies have been able to identify genetic variants linked to IBS.20–22
Determining the contributions of genetic and family environmental factors is difficult in family studies of IBS. This is because most children, including dizygotic (DZ) and monozygotic (MZ) twins, grow up in their biological families.10 11 An important assumption in twin studies is that MZ and DZ twins show similarities because of shared environmental factors so that the difference in concordance rates between MZ and DZ twins is only a reflection of genetic factors.11 However, studies suggest that MZ twins are treated more similarly than DZ twins, which theoretically may inflate the estimated heritability determined in twin studies.23 It may therefore be of value to have other methods than twin studies as a determinant of the heritability for IBS. Studying adoptees is an appropriate alternative for analysing the genetic and shared familial environmental influence on the transmission of IBS.11 24 25 Studies of adoptees offer an opportunity to understand the genetic transmission of IBS because adoptees do not grow up in their biological families.11 Transmission of IBS from biological parents to offspring would therefore be explained by biological (genetic factors) or early life factors rather than family environment. In addition, transmission of IBS from adoptive parents to their non-biological offspring would be explained by family environment rather than genetic factors. To the best of our knowledge, no study has examined the familial aggregation in adoptees with IBS with the aim to shed new light on the familial transmission of IBS.
This study used the Swedish Inpatient Register, the Swedish Outpatient Care Register, a Swedish Primary Healthcare Register and the Swedish Multigeneration Register. Our study had two primary aims: (1) to examine the risk and heritability of IBS in adoptees with a biological parent affected by IBS and (2) to examine the risk of IBS in adoptees with an adoptive parent affected by IBS.
Methods
We linked comprehensive registers and nationwide healthcare data from multiple sources to assess IBS among individuals in Sweden.26–31 This linkage was based on the unique individual Swedish 10-digit personal ID numbers assigned at birth or immigration to all residents in Sweden for life. This information is nearly 100% complete. These numbers were replaced with serial numbers to preserve anonymity. We used data from the following sources:
The Swedish Multigeneration Register; this contains information on family relationships including adoptions. The register contains information on index persons registered in Sweden from 1 January 1961 and born from 1 January 1932 onwards.
The Lisa Register from Statistics Sweden (SCB), which contains annual data on education status from 1990 to 2012. It also contains the Swedish Standard Classification of Occupations 1996, which is a national version of the International Standard Classification of Occupations.
The Swedish Hospital Discharge Register, which contains all hospital diagnoses for all people in Sweden from 1964 to 2012. The register has had nationwide coverage since 1987.
The Hospital Outpatient Care Register, which contains information on diagnoses from all specialist outpatient clinics in Sweden from 2001 to 2012.
The Swedish Cause of Death Register, which contains data on date and cause of death from 1964 to 2012.
A nationwide Primary Healthcare register, which contains data from 1989 to 2016 (with 7 908 367 individuals in registers from 12 regions) (see online supplementary tables 1 and 2 and supplementary figure 1).
The Migration register, which contains data on immigration and emigration from 1892 to 2012.
Census registers, including individual addresses, available every 5 years between 1960 and 1990.
From 1991 Small Area Market Statistics (SAMS) data has been used to define a municipal subarea when you need to characterise a neighbourhood; the code comprises the county, the municipality and unique SAMS area (9200 in whole Sweden). Neighbourhood Deprivation Index (NDI) was created according to Winkleby et al and was based on educational status; income; unemployment and social welfare recipient.32 A z score was calculated for each SAMS neighbourhood. The z scores, weighted by the coefficients for the eigenvectors, were then summed to create the index. The index was categorised into three groups: below 1 SD from the mean (low deprivation), above 1 SD from the mean (high deprivation) and within 1 SD of the mean (moderate deprivation). Higher scores reflect more deprived neighbourhoods.32
bmjgast-2017-000156supp001.docx (26.2KB, docx)
Study approval
The study was approved by the Ethics Committee of Lund University, Sweden, and was performed in compliance with the Helsinki Declaration. Informed consent was waived as a requirement by the ethics committee.
Definition of IBS
Cases of IBS in the Swedish Hospital Discharge Register, Outpatient Care Register and Primary Healthcare register were identified by the following International Classification of Diseases (ICD) codes: ICD-7 573.10, 573.21, 573.22; ICD-8 564.10, 564.11, 564.19; ICD-9 564B (IBS) and ICD-10 K58 (IBS). Main and all secondary diagnoses were used. The validity in the Hospital Discharge Register is generally 85%–95%.30 The present study may not be representative of all patients with IBS in Sweden and may introduce a selection bias as the diagnosis of IBS is based on healthcare seeking.33 However, familial risk in Sweden is similar using these national specialist register and primary healthcare data.9 We excluded patients with IBS with possible gastrointestinal differential diagnosis, that is, coeliac disease, inflammatory bowel disease (IBD) and colorectal cancer. ICD codes are presented in online supplementary tables 3; and (5) adoptees not linked to at least one biological and at least one adoptive parent.9
Sample
The analyses were based on a dataset containing information on the entire Swedish population, including parental relationships. The dataset contains all Swedish-born children that were adopted (born 1951–1995) with respective biological or adoptive parents. We excluded adoptees from the study if they had: (1) died before age 16 years (death year–birth year); (2) migrated from Sweden before age 16 years (migration year–birth year); (3) died before 1964; (4) gastrointestinal differential diagnosis, that is, patients with IBS with coeliac disease, IBD and colorectal cancer were excluded. ICD codes are presented in online supplementary tables 3; and (5) adoptees not linked to at least one biological and at least one adoptive parent. All adoptive children who had lived with a biological parent were excluded according to Census (1960–1990) or SAMS (from 1991). For those born between 1951 and 1959, the status in the 1960 census was used.
We also excluded adoptees that had lived with their adoptive grandparent, aunt/uncle and sibling or with step-parents and their biological parent. A total of 30 693 adoptees remained in the study after exclusions. They constitute the study population in the cohort study. These adoptees could be linked to 51 634 adoptive parents and 49 912 biological parents.
After exclusions, we identified 2288 (1.73%) IBS cases. A total of 776 IBS cases were found in adoptees, 840 IBS cases in biological parents and 660 IBS cases in adoptive parents. Of the 2288 IBS cases, 55.07% (1260) were found in the Primary Healthcare register and 44.93% (1028) in the Hospital register. Among the hospital-diagnosed IBS cases, 330 (32.10%) were from the Hospital Discharge register n=330 and 698 (67.90%) from the specialist Outpatient register. Of all IBS cases, 5.68% (n=130) were identified with ICD-8, 3.63% (n=83) with ICD-9 and 90.69% (n=2075) with ICD-10. No case was identified with ICD-7.
Statistical calculations
We collected data on adoptees and their biological and adoptive parents from 1964 to 2012 in order to assess the genetic and environmental influences in IBS disease. We used a cohort design and a case-control approach. We conducted two main analyses: one using biological parents and one using adoptive parents. We used case-control exact matching method (1:5) by drawing a sample of affected adoptees as cases and matched control groups of unaffected adoptees.34 The control groups were matched based on sex, birth year, county of birth and level of education. In the case-control study, we connected both groups using connection codes to their biological and adoptive parents.35 For the case-control study, analyses were conducted using conditional logistic regression. For the cohort study, we used logistic regression. In the multivariate model, we used adoptees' birth year, sex, education of adoptees and county (region) of birth of adoptees as covariates. The estimated parameters were odds of an adoptee to IBS when at least one biological parent had got IBS relative to the odds of an adoptee to IBS when no biological parents had IBS, and similarly for adoptive parents. We also created a new age-stratified category variable based on an adopted child’s age distribution after matching.
We used Falconer’s regression, which is based on the liability of the threshold, to obtain heritability in adoptees of the biological parents.36 Using the prevalence rate of the relatives of the biological probands and the controls from the case-control study, the heritability h 2 (and ±SE) was calculated.36 We also used the approach described by Frisell et al to evaluate heritability.37 Using the case-control procedure, we calculated tetrachoric correlations and heritability, according to the prevalence in the present cohort study and for a wide range of different population prevalences of IBS.37 Under the assumption that only additive genetic factors contribute to similarity among relatives without any shared familial environment, the heritability of liability may be estimated as twice the observed tetrachoric correlation among first-degree relatives according to Falconer and Mackay.38
Statistical analysis was performed with SAS V.9.3 (SAS Institute) and for calculating heritability, we used R software (V.3.3.2). A level of p<0.05 (two-sided) was considered statistically significant.
Results
Descriptive statistics
During the study period (1951–1995), a total of 2288 individuals were diagnosed with IBS (excluding individuals with a concomitant coeliac disease, IBD and colorectal cancer). Table 1 shows the descriptive statistics for adopted offspring, biological parents and adoptive parents, that is, age, sex, educational attainments, NDI, occupation, IBS and age at IBS diagnosis and age at end of follow-up. Cases of IBS were more often found among females. The prevalence of IBS among biological parents was 1.68% (840/49 912), while among the adoptive parents it was 1.28% (660/51 634). Thus, there was no statistically significant difference between these groups (X2=1.42, p=0.23). The adoptive parents with median age of 76 years (IQR 63–83 years) were older than biological parents with a median age of 68 years (IQR=60–75 years) at end of follow-up. Table 2 shows that the median birth year of adoptees was 1963 (IQR 1957–1968), for biological parents it was 1939 (IQR 1932–1946), while it was 1928 (IQR 1921–1938) for adoptive parents. The age distribution for Swedish born (1951–1995) adoptees at first time diagnosis of IBS is shown in figure 1. Biological parents also had lower education, lived in more deprived neighbourhoods and less often had an occupation with a requirement for in-depth university competence. In table 3, non-affected adoptees are compared with affected adoptees. Affected adoptees were significantly more often females (p<0.0001) and less often had an occupation with a requirement for in-depth university competence (p=0.009).
Table 1.
Descriptive statistics of 30 693 adoptees and their adoptive (n=51 634) and biological parents 49 912 (132 239 individuals in total)
| Adoptees (n=30 693) |
Adoptive parents (n=51 634) |
Biological parents (n=49 912) |
|
| Sex* | |||
| Female | 14 883 (48.49%) | 22 547 (43.67%) | 29 706 (59.52%) |
| IBS* | 776 (2.53%) | 660† (1.28%) | 840‡ (1.68%) |
| Female | 552 (1.80) | 433 (0.84) | 693 (1.39) |
| High education* (12 years or more) | 9004 (29.34%) | 9067 (17.67%) | 4973 (9.96%) |
| NDI (high socioeconomic status) | 407 (1.33%) | 4575 (8.86%) | 2426 (4.86%) |
| Occupation§ | 5775 (18.82%) | 5832 (11.29%) | 3475 (6.96%) |
| Age at IBS diagnosis (median and IQR) |
43 (35–49) | 71 (63–78) | 62 (55–69) |
| Age at end of follow-up (median and IQR) |
49 (43–54) | 76 (68–83) | 68 (60–75) |
*Number of observations (%).
†Four adoptees had two adoptive parents with IBS.
‡Eight adoptees had two biological parents with IBS.
Chief or occupation with a requirement for in-depth university competence.
IBS, irritable bowel syndrome; NDI, Neighbourhood Deprivation Index.
Table 2.
The distribution of the birth years for adoptees and their adoptive and biological parents are shown
| n | Minimum | Maximum | Mean | SD | Median | Q1–Q3 | |
| Adopted offspring | 30 693 | 1951 | 1995 | 1964 | 9 | 1963 | 1957–1968 |
| Adoptive parents | 51 634 | 1888 | 1979 | 1930 | 12 | 1928 | 1921–1938 |
| Biological parents | 49 912 | 1884 | 1980 | 1939 | 11 | 1939 | 1932–1946 |
Q1–Q3=IQR range.
Figure 1.
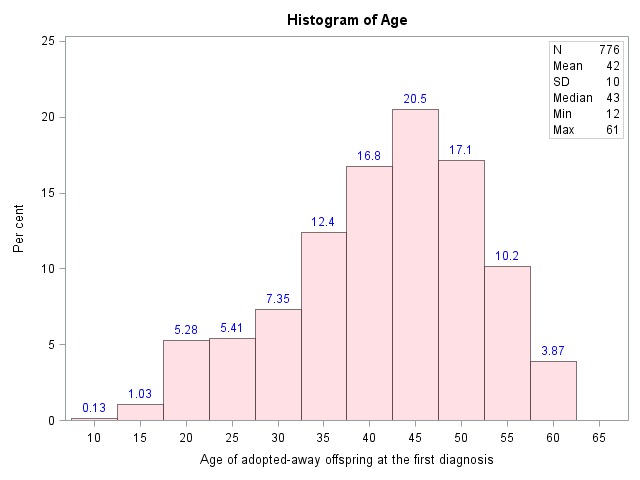
Age distribution for Swedish born (1951–1995) adoptees at first time diagnosis of irritable bowel syndrome.
Table 3.
Descriptive statistics of 30 693 adoptees with and without diagnosis of IBS
| No IBS (n=29 917) |
IBS (n=776) |
p Value | |
| Sex | |||
| Female | 14 331 (47.90%) | 552 (71.13%) | <0.0001* |
| High education (12 years or more) | 8756 (29.27%) | 248 (31.96%) | 0.104* |
| NDI (high socioeconomic status) | 403 (1.35%) | 4 (0.52%) | 0.053† |
| Occupation‡ | 5657 (18.91%) | 118 (15.21%) | *0.009 |
| Age at end of follow-up (years) (median and IQR) |
49 (43–54) | 48 (43–54) | 0.239§ |
*Χ2 test.
†Fisher’s exact test.
‡Chief or occupation with a requirement for in-depth university competence.
§Wilcoxon test.
IBS, irritable bowel syndrome; NDI, Neighbourhood Deprivation Index.
Cohort design
The estimated OR with 95% CI in the cohort design is shown in table 4. In the crude model, the OR for IBS in adoptees of biological parents of which at least one had IBS was increased, OR 1.66 (95% CI 1.17 to 2.35). The OR in the adjusted model (model 2) was also significantly increased, OR 1.63 (95% CI 1.14 to 2.32). The estimated OR for IBS in adoptees with an affected adoptive parent was not significantly increased either in the crude model (OR 0.77; 95% CI 0.44 to 1.34) or in the adjusted model (OR 0.75; 95% CI 0.43 to 1.329).
Table 4.
OR determined with logistic regression for IBS in adoptees with an affected biological or adoptive parent (cohort design)
| Risk factors | Ref | Biological parents | Adoptive parents | ||
| Model 1* | Model 2† | Model 3* | Model 4† | ||
| IBS | 0 | 1.66 (1.17–2.35) | 1.63 (1.14–2.32) | 0.77 (0.44–1.34) | 0.75 (0.43–1.32) |
| Year of birth | 1.01 (1.00–1.02) | 1.01 (1.00–1.02) | 1.01 (1.00–1.02) | 1.01 (1.00–1.02) | |
| Sex | Male | 2.68 (2.29–3.14) | 2.61 (2.23–3.06) | 2.68 (2.29–3.14) | 2.61 (2.23–3.06) |
| County (region) | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) | |
| Education | 1.25 (1.13–1.39) | 1.16 (1.04–1.29) | 1.25 (1.13–1.39) | 1.16 (1.04–1.29) | |
*Univariate model.
†Multivariate model.
IBS, irritable bowel syndrome; Ref, reference.
Case-control study
The results of the case-control study are shown in table 5. IBS in the adoptees was significantly associated with IBS in biological parents with an OR of 1.67 (95% CI 1.06 to 2.62) in adoptees with an affected biological parent. IBS in an adoptive parent was not significantly associated with IBS in adoptees (OR 0.88 (95 % CI 0.48 to 1.63)). The age-stratified ORs were not significantly increased.
Table 5.
Results for the matched case-control study (1:5)
| All* | Age≤45 years† | Age>45 years‡ | |
| ORs for IBS in adoptees with an affected biological parent | 1.67 (1.06 to 2.62) | 1.70 (0.93 to 3.08) | 1.63 (0.82 to 3.25) |
| ORs for IBS in adoptees with an affected adoptive parent | 0.88 (0.48 to 1.63) | 1.03 (0.48 to 2.21) | 0.69 (0.24 to 1.96) |
ORs for IBS among adoptees with an affected biological or adoptive parent. Age-stratified ORs for IBS are also shown. Data are presented as OR (95% CI).
*Cases (n=569) and controls (n=2 845).
†Cases (n=315) and controls (n=1 575).
‡Cases (n=254) and controls (n=1 270).
IBS, irritable bowel syndrome.
Heritability
By using Falconer’s method, we obtained the estimated heritability (h 2) in biological parents of adoptees with IBS. The heritability h 2 for IBS calculated from the case-control study was 19.5%±8.5%. The heritability was also determined by tetrachoric correlation in the case-control study with different estimates of the population prevalence of IBS (table 6). We did not know the prevalence in the particular source population exactly but based on previous studies we were able to choose a range of likely values and present a corresponding range of heritability estimates. The results are presented in table 6. The heritability varied from 16% in a population with 0.5% prevalence to 27% in a population with 20% prevalence. With a prevalence of 1.73% (table 1), as in the present population, the heritability was 18.3%.
Table 6.
Heritability of irritable bowel syndrome based on estimated population prevalence and tetrachoric correlation in case-control study according to Frisell et al 37
| Exposed cases | Unexposed cases | OR | Prevalence | Tetrachoric correlation | Heritability (%) |
| 26 | 543 | 1.67 | 0.5 | 0.08 | 16 |
| 26 | 543 | 1.67 | 1.0 | 0.09 | 17 |
| 26 | 543 | 1.67 | 3.0 | 0.10 | 20 |
| 26 | 543 | 1.67 | 5.0 | 0.11 | 22 |
| 26 | 543 | 1.67 | 10.0 | 0.12 | 24 |
| 26 | 543 | 1.67 | 15.0 | 0.125 | 25 |
| 26 | 543 | 1.67 | 20.0 | 0.133 | 27 |
Discussion
This is the first study of IBS in adoptees and their biological and adoptive parents. An association was found between IBS disease in adoptees and their biological but not adoptive parents. The OR estimated in the present study is lower than among first-degree relatives that are not adopted according to previous published studies,5–9 which suggests a contribution of familial environmental factors. However, familial environmental factors on their own are not enough to cause IBS among adoptees. IBS in adoptive parents does not increase the odds of IBS in adoptive children. The heritability h 2 could also be estimated among adoptees in the present study and was determined to be 19.5%±8.5% with Falconer’s method and between 16% and 27% tetrachoric correlations depending on the prevalence of IBS in the population. These numbers are close to several published twin studies, although the heritability in published twin studies varies from 19% to 48%.12–19 The present study adds to increasing evidence for genetic factors being important in IBS.20–22 Recently genetic variants have been associated with IBS.21 22
The present study cannot rule out that shared environmental factors are of importance. Most adoptees who were diagnosed with IBS at first time were adults. We do not know whether any possible effects of familial environmental factors are weakened or not after adoptees become adults and move from their adoptive parents. Previously, an increased risk of IBS has been observed among spouses, which suggests an effect of shared adult familial environment.9
Strength of this study is that we used nationwide specialist care registers and a large primary healthcare database containing information on all primary healthcare visits from well-defined areas. This approach minimised any selection bias. A limitation of our present study is that we did not have access to how diagnosis of IBS was determined. However, the prevalence is low and similar to previously published Swedish register-based studies.9 32 A limitation is that we do not know whether the Rome criteria were followed or not. Moreover, the criteria for IBS have also changed over time. IBS has not been evaluated in the present register but IBS diagnoses have been evaluated in an English primary healthcare register with a positive predictive value of 77%.39 The sex and age distribution is as expected in an IBS population.1–3 This may indirectly suggest that the ICD code mostly identifies patients with IBS in the used registers. However, it is possible that those seeking healthcare are the most severely affected cases. This might be an advantage in genetics because there are usually more genetic factors in more severe cases in complex traits, which could be an advantage of the present study.4 The study population is limited to Swedish-born adoptees and is therefore only valid for Caucasians.
In conclusion, the present study shows that biological (genetic) factors are important in the familial transmission of IBS. We have also, in a novel way, determined the heritability with results that confirm twin studies, which suggests that future studies of genetics of IBS will be fruitful.
Acknowledgments
The authors wish to thank the CPF’s science editor Patrick Reilly for his useful comments on the text. The registers used in the present study are maintained by Statistics Sweden and the National Board of Health and Welfare.
Footnotes
Contributors: RW, BZ, JS, KS and MP were involved in study design and execution, and finalising the paper. RW and BZ drafted the manuscript. All authors critically revised the paper and all authors approved the final draft submitted. All authors had full access to all the data (including statistical reports and tables) and take responsibility for the integrity of the data and the accuracy of their analysis.
Funding: This work was supported by grants to BZ and KS and JS from the Swedish Research Council, ALF funding awarded to BZ, KS and JS, and the Swedish Heart-Lung Foundation (BZ).
Competing interests: None declared.
Ethics approval: Lund University.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The nationwide registers used in the present study are maintained by Statistics Sweden and the National Board of Health and Welfare.
References
- 1. Longstreth GF, Thompson WG, Chey WD, et al. . Functional bowel disorders. Gastroenterology 2006;130:1480–91. doi:10.1053/j.gastro.2005.11.061 [DOI] [PubMed] [Google Scholar]
- 2. Khan S, Chang L. Diagnosis and management of IBS. Nat Rev Gastroenterol Hepatol 2010;7:565–81. doi:10.1038/nrgastro.2010.137 [DOI] [PubMed] [Google Scholar]
- 3. Saito YA, Schoenfeld P, Locke GR. The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol 2002;97:1910–5. doi:10.1111/j.1572-0241.2002.05913.x [DOI] [PubMed] [Google Scholar]
- 4. Lander ES, Schork NJ. Genetic dissection of complex traits. Science 1994;265:2037–48. doi:10.1126/science.8091226 [DOI] [PubMed] [Google Scholar]
- 5. Whorwell PJ, McCallum M, Creed FH, et al. . Non-colonic features of irritable bowel syndrome. Gut 1986;27:37–40. doi:10.1136/gut.27.1.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kalantar JS, Locke GR, Zinsmeister AR, et al. . Familial aggregation of irritable bowel syndrome: a prospective study. Gut 2003;52:1703–7. doi:10.1136/gut.52.12.1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saito YA, Zimmerman JM, Harmsen WS, et al. . Irritable bowel syndrome aggregates strongly in families: a family-based case-control study. Neurogastroenterol Motil 2008;20:790–7. doi:10.1111/j.1365-2982.2007.01077.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saito YA, Petersen GM, Larson JJ, et al. . Familial aggregation of irritable bowel syndrome: a family case-control study. Am J Gastroenterol 2010;105:833–41. doi:10.1038/ajg.2010.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Waehrens R, Ohlsson H, Sundquist J, et al. . Risk of irritable bowel syndrome in first-degree, second-degree and third-degree relatives of affected individuals: a nationwide family study in Sweden. Gut 2015;64:215–21. doi:10.1136/gutjnl-2013-305705 [DOI] [PubMed] [Google Scholar]
- 10. Burton PR, Tobin MD, Hopper JL. Key concepts in genetic epidemiology. Lancet 2005;366:941–51. doi:10.1016/S0140-6736(05)67322-9 [DOI] [PubMed] [Google Scholar]
- 11. Risch N. The genetic epidemiology of cancer: interpreting family and twin studies and their implications for molecular genetic approaches. Cancer Epidemiol Biomarkers Prev 2001;10:733–41. [PubMed] [Google Scholar]
- 12. Morris-Yates A, Talley NJ, Boyce PM, et al. . Evidence of a genetic contribution to functional bowel disorder. Am J Gastroenterol 1998;93:1311–7. doi:10.1111/j.1572-0241.1998.440_j.x [DOI] [PubMed] [Google Scholar]
- 13. Levy RL, Jones KR, Whitehead WE, et al. . Irritable bowel syndrome in twins: heredity and social learning both contribute to etiology. Gastroenterology 2001;121:799–804. doi:10.1053/gast.2001.27995 [DOI] [PubMed] [Google Scholar]
- 14. Mohammed I, Cherkas LF, Riley SA, et al. . Genetic influences in irritable bowel syndrome: a twin study. Am J Gastroenterol 2005;100:1340–4. doi:10.1111/j.1572-0241.2005.41700.x [DOI] [PubMed] [Google Scholar]
- 15. Bengtson MB, Rønning T, Vatn MH, et al. . Irritable bowel syndrome in twins: genes and environment. Gut 2006;55:1754–9. doi:10.1136/gut.2006.097287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lembo A, Zaman M, Jones M, et al. . Influence of genetics on irritable bowel syndrome, gastro-oesophageal reflux and dyspepsia: a twin study. Aliment Pharmacol Ther 2007;25:1343–50. doi:10.1111/j.1365-2036.2007.03326.x [DOI] [PubMed] [Google Scholar]
- 17. Svedberg P, Johansson S, Wallander MA, et al. . No evidence of sex differences in heritability of irritable bowel syndrome in Swedish twins. Twin Res Hum Genet 2008;11:197–203. doi:10.1375/twin.11.2.197 [DOI] [PubMed] [Google Scholar]
- 18. Nielsen CS, Knudsen GP, Steingrímsdóttir ÓA. Twin studies of pain. Clin Genet 2012;82:331–40. doi:10.1111/j.1399-0004.2012.01938.x [DOI] [PubMed] [Google Scholar]
- 19. Vehof J, Zavos HM, Lachance G, et al. . Shared genetic factors underlie chronic pain syndromes. Pain 2014;155:1562–8. doi:10.1016/j.pain.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 20. Camilleri M. Genetics and irritable bowel syndrome: from genomics to intermediate phenotype and pharmacogenetics. Dig Dis Sci 2009;54:2318–24. doi:10.1007/s10620-009-0903-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ek WE, Reznichenko A, Ripke S, et al. . Exploring the genetics of irritable bowel syndrome: a GWA study in the general population and replication in multinational case-control cohorts. Gut 2015;64:1774–82. doi:10.1136/gutjnl-2014-307997 [DOI] [PubMed] [Google Scholar]
- 22. Henström M, Diekmann L, Bonfiglio F, et al. . Functional variants in the sucrase-isomaltase gene associate with increased risk of irritable bowel syndrome. Gut 2016. (Epub ahead of print). doi:10.1136/gutjnl-2016-312456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haworth CM, Dale P, Plomin R. A Twin study into the genetic and environmental influences on academic performance in science in nine-year-old boys and girls. Int J Sci Educ 2008;30:1003–25. doi:10.1080/09500690701324190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kendler KS, Larsson Lönn S, Morris NA, et al. . A Swedish national adoption study of criminality. Psychol Med 2014;44:1913–25. doi:10.1017/S0033291713002638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kendler KS, Ji J, Edwards AC, et al. . An extended Swedish national adoption study of alcohol use disorder. JAMA Psychiatry 2015;72:211–8. doi:10.1001/jamapsychiatry.2014.2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Statistics Sweden. [The Swedish Multigeneration Register (1960–1990)] Registret över totalbefolkningen/RTB (In Swedish). Stockholm: Statistics Sweden, 2005. [Google Scholar]
- 27. The National Board of Health and Welfare. [Validity of the diagnoses from the Swedish In-Care Register 1987 and 1995] (In Swedish). Stockholm: Epidemiologiskt Centrum, the National Board of Health and Welfare, 2000. [Google Scholar]
- 28. Rosen M, Hakulinen T. Use of disease registers Handbook of epidemiology. Berlin: Springer-Verlag, 2005:231–51. [Google Scholar]
- 29. Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, et al. . The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 2009;24:659–67. doi:10.1007/s10654-009-9350-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ludvigsson JF, Andersson E, Ekbom A, et al. . External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450 doi:10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zöller B. Nationwide family studies of cardiovascular diseases—clinical and genetic implications of family history. EMJ Cardiology 2013;1:102–13. [Google Scholar]
- 32. Winkleby M, Sundquist K, Cubbin C. Inequities in CHD incidence and case fatality by neighborhood deprivation. Am J Prev Med 2007;32:97–106. doi:10.1016/j.amepre.2006.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Waehrens R, Ohlsson H, Sundquist J, et al. . Low prevalence of irritable bowel syndrome in primary health care in four Swedish counties. Scand J Prim Health Care 2013;31:132–7. doi:10.3109/02813432.2013.811949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thomas DC. Statistical methods in genetic epidemiology. Oxford: Oxford University Press, 2004. [Google Scholar]
- 35. William YR. Adoption studies. Hoboken, NJ: John Wiley & Sons, Inc, 2011. [Google Scholar]
- 36. Falconer DS. The inheritance of liability to certain diseases, estimated from the incidence among relatives. Ann Hum Genet, Lond 1965;29:51–76. doi:10.1111/j.1469-1809.1965.tb00500.x [Google Scholar]
- 37. Frisell T, Holmqvist M, Källberg H, et al. . Familial risks and heritability of rheumatoid arthritis: role of rheumatoid factor/anti-citrullinated protein antibody status, number and type of affected relatives, sex, and age. Arthritis Rheum 2013;65:2773–82. doi:10.1002/art.38097 [DOI] [PubMed] [Google Scholar]
- 38. Falconer DS, Mackay TF. Introduction to quantitavive genetics. 4th edn Harlow, England: Pearson Educated Limited, 1996. [Google Scholar]
- 39. Khan NF, Harrison SE, Rose PW. Validity of diagnostic coding within the general practice research database: a systematic review. Br J Gen Pract 2010;60:128–36. doi:10.3399/bjgp10X483562 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgast-2017-000156supp001.docx (26.2KB, docx)


