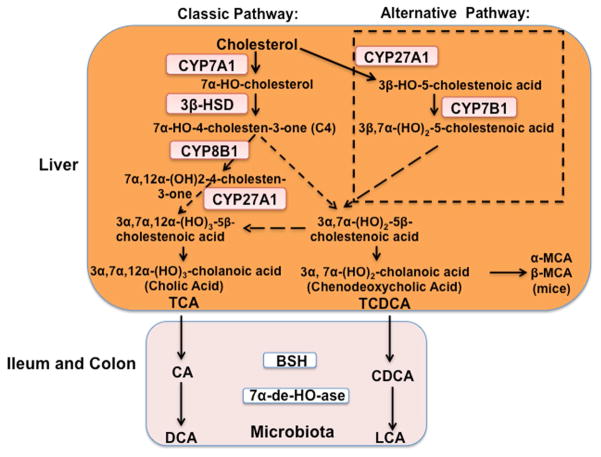
Fig. 1. Bile acid synthesis pathways.
Two bile acid synthesis pathways are involved in the conversion of cholesterol to bile acids in the liver. The classic pathway is initiated by cholesterol 7α-hydroxylase (CYP7A1), and the alternative pathway is initiated by steroid 27-hydroxylase (CYP27A1). 3β-hydroxysteroid dehydrogenase (3β-HSD) converts 7α-hydroxycholesterol to 7α-hydroxy-4-cholesten-3-one (C4). Serum C4 level has been used as a marker for the rate of bile acid synthesis. Sterol 12-hydroxylase (CYP8B1) is a branch enzyme that synthesizes cholic acid (CA). Without 12α-hydroxylation, chenodeoxycholic acid (CDCA) is synthesized. Mitochondrial CYP27A1 catalyzes oxidation of the steroid side chain, and the peroxisomal β-oxidation reaction cleaves a 3C unit to form C24 cholestenoic acid, the backbone of most bile acids. CA and CDCA are the two primary bile acids synthesized in human liver. In mice, CDCA is converted to α- and β-muricholic acids (α-MCA and β-MCA, respectively). Bile acids are immediately conjugated to the amino acids taurine or glycine (TCA or TCDCA, respectively) for secretion into bile. In the ileum, TCA and TCDCA are deconjugated by bacterial bile salt hydrolase (BSH) activity, and the 7α-hydroxyl group is removed by bacterial 7α-dehydroxylase activity to form deoxycholic acid (DCA) and lithocholic acid (LCA), respectively. Bile acids (TCA, TDCA, TCDCA, Tα-MCA, and Tβ-MCA) are re-conjugated and circulated back to the liver. LCA is secreted into feces, and a small amount is circulated to the liver, conjugated to sulfite, and secreted into urine.