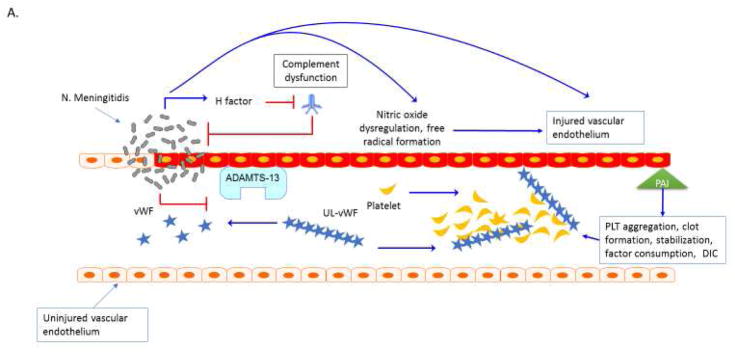
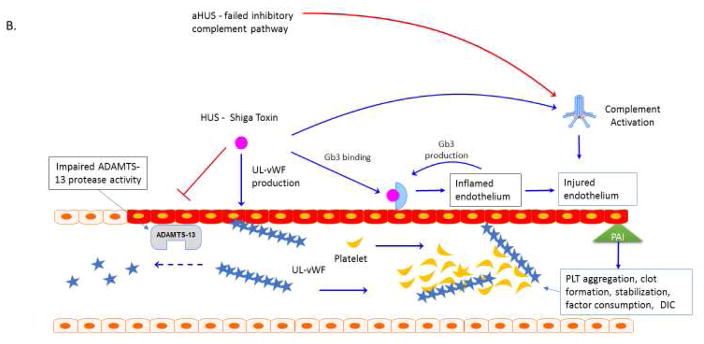
Figure 1. Thrombocytopenia Associated MOF. A. Purpura fulminans.
Neisseria meningitidis can adhere to and form colonies on the vascular endothelium via pilin62. Meningococcal virulence factor such as factor H binding protein can down regulate complement response and allow bacterial proliferation63. Bacterial-endothelial interactions result in endothelial inflammation via NFkB responses and can further promote endothelial infection, capillary leakage and translocation of bacteria across the vessel64–66. Invasive meningococcal disease is associated with decreased activity of the metalloproteinase ADAMST13 and increased activity of von Willebrand factor (vWF) 67, 68. Ultra-large vWF (UL vWF) multimers contribute to platelet (PLT) aggregation and intravascular thrombosis68,69. Endothelial inflammation contributes to platelet dysfunction via N. meningitidis-derived nitric oxide with impaired vascular homeostasis and NO mediated impairment ADP-mediated platelet aggregation70. Inflammatory cytokines change the coagulation profile towards pro-coagulation with a reduction of activated protein C (APC) and anti-thrombin III (ATIII) and upregulation of prothrombin and the anti-fibrinolytic plasminogen activator inhibitor-1 (PAI-1)71. The end result of the interaction of multiple ‘inflammatory’ pathways in the organ is micro-thrombosis, tissue ischemia, oxidative insult, ischemia and cell death. Plasm exchange therapy can reverse this process. B. Atypical Hemolytic Uremic Syndromes. Sterile microangiopathies such as thrombotic thrombocytopenia purpura (TTP) are associated with inhibition of ADAMST13 activity via the presence of auto-inhibitors, whereas atypical HUS or “congenital TTP” has been associated with ADAMST13 and inhibitory complement gene mutations72,73. Upon infection with Shiga toxin (ST)-producing pathogens, ST has direct effects on the vascular endothelium and results in increased release of ultra-large vWF multimers and to directly inhibit ADAMST13 activity level, further promoting thrombosis formation73–74. ST mediated aHUS causes a pro-inflammatory endothelial state by promoting leukocyte adhesion and by producing endothelial derived cytokines such as IL-8, similar to the vascular pathophysiology observed in purpura fulminans. Exposure of human endothelial cells to the pro-inflammatory cytokines TNF-al and IL-1b increase expression of globotriaosylceramide (Gb3) on endothelial cells and result in further susceptibility to ST by a feed-forward mechanism75. Shiga-toxin causes production of Complement 3a (C3a), with loss of thombomodulin (TM), changes in cell surface adhesion molecules and a propensity toward clot production2, 76–78. In aHUS, mutations in the alternative complement regulatory pathway, especially mutations in the complement factor H, impair control of Complement 3b (C3b) on the cell surface by inability to recognize sialic acid and helps to explain the thrombogenic potential in these patients2, 79. Mutations have recently been described in multiple complement regulatory genes in adult patients with aHUS, with 12% of patients having compound mutations80. In addition to plasma exchange therapy, the anti-C5a monoclonal antibody eculizumab is FDA approved for this process.
Abbreviations: Thombocytopenia Associated Multiple Organ Failure (TA-MOF); Nuclear factor kappa b (NFkb); A disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMST13); von Willebrand factor (vWF), Platelet (PLT); Nitric Oxide (NO); Activated protein C (APC); Anti-thrombin III (ATIII); plasminogen activator inhibitor-1 (PAI-1); thrombotic thrombocytopenia purpura (TTP); hemolytic uremic syndrome (HUS); atypical HUS (aHUS); shiga toxin (ST); tumor necrosis factor alpha (TNF-a); interleukin 1 beta (IL-1b); globotriaosylceramide (Gb3); complement 3a (C3a); thrombomodulin (TM), complement 3b (C3b).