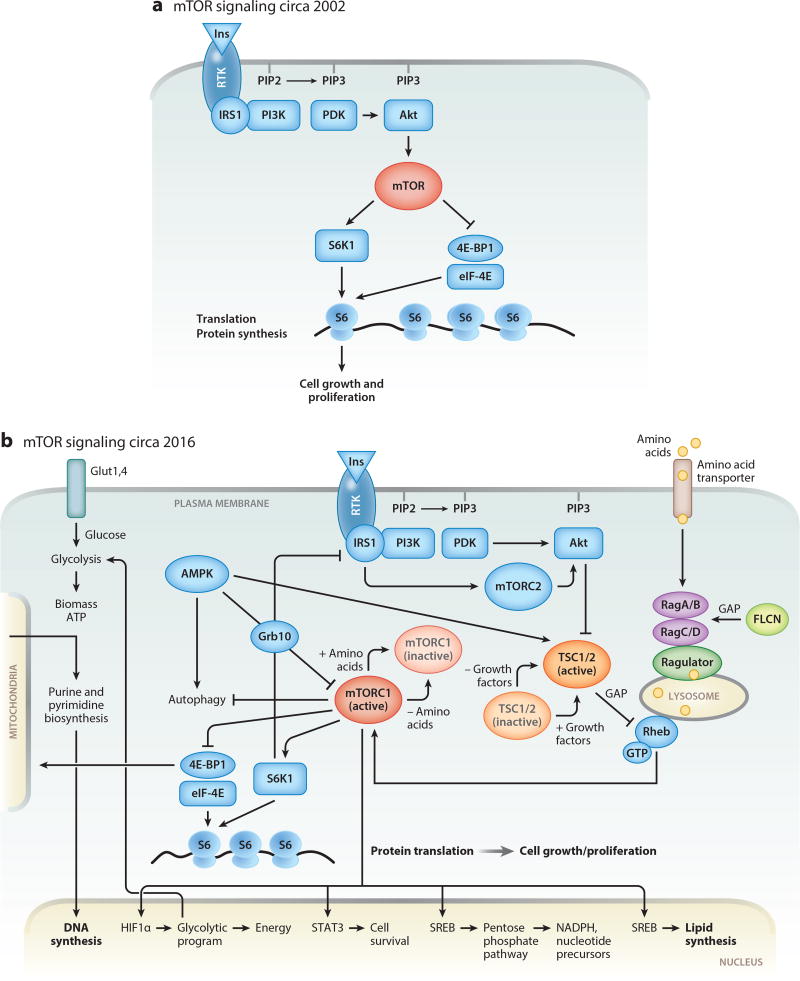
Figure 1.
(a) Schematic representation of the mTOR signaling pathway before discovery of TSC function. (b) Simplified schematic representation of our current understanding of the mTOR signaling pathway. In normal cells, growth factors or insulin (Ins) activate their cognate receptor tyrosine kinases (RTKs), leading to autophosphorylation. Activated RTKs recruit PI3K, a key enzyme that transduces extracellular signaling into cellular responses by activating serine-threonine kinase Akt. Akt phosphorylates TSC (a complex of two proteins, TSC1 and TSC2), releasing the small GTPase Rheb from TSC suppression, which results in activation of mTORC1. In lymphangioleiomyomatosis (LAM), the TSC1/2 complex is mutationally inactivated and does not suppress Rheb. Activated Rheb and amino acids, through the complex composed of Ragulator, RagA/B, and RagC/D, recruit mTORC1 to the lysosomal surface. Constitutively activated mTORC1 induces metabolic reprogramming by (1) upregulating transcription of HIF1α, thereby modulating the expression of glycolytic enzymes, SREBP transcription factors mediating synthesis of fatty acids and cholesterol, and enzymes of the pentose phosphate pathway (PPP); (2) increasing translation of ribosomal and mitochondrial proteins, thereby inducing ribosomal and mitochondrial biogenesis; and (3) inducing de novo synthesis of proteins, lipids, and nucleotides (purines and pyrimidines). STAT3 is required for LAM cell survival, consistent with a role for the mTORC1-STAT3 axis in regulation of prosurvival genes in TSC1/2-deficient cells.