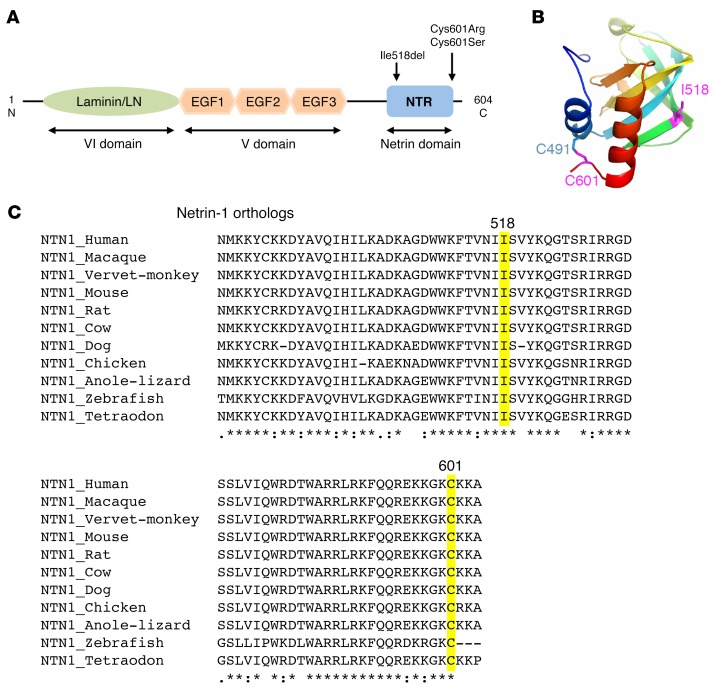
Figure 2. Position and conservation of the mutated amino acids.
(A) Schematic of the netrin-1 protein showing the laminin (LN) domain, the 3 EGF-like domains, and the netrin (NTR) domain. Arrows show the location of the 3 mutations. (B) Structural model of the NTR domain of WT netrin-1 showing the 2 cysteines involved in a disulfide bridge (C601 and C491) and I518, which has a key position in a β-strand. The 2 mutated amino acids (I518 and C601) are in purple. (C) Alignment of the regions flanking the 3 variants in vertebrate netrin-1 orthologous proteins, showing the conservation of the altered amino acids. Multiple pairwise alignments were performed using Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/). The amino acids altered by the mutations are highlighted. Asterisk indicates a fully conserved residue; colon indicates conservation between groups of strongly similar properties; period indicates conservation between groups of weakly similar properties.