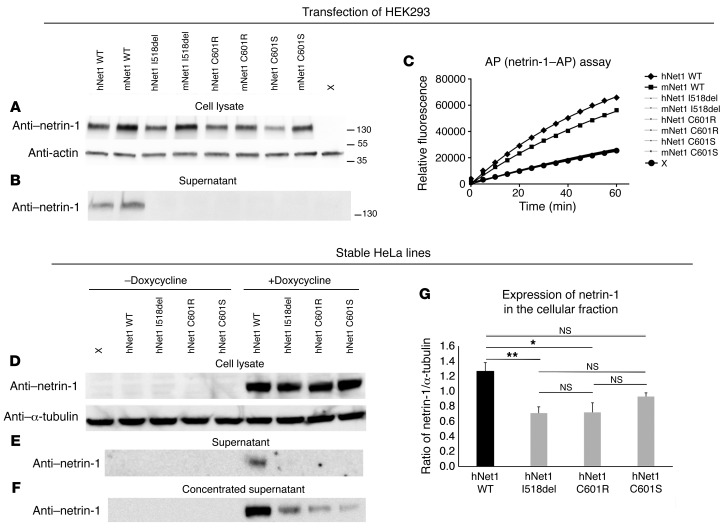
Figure 5. Expression of the WT and mutated netrin-1–AP and netrin-1 constructs.
(A–C) HEK293 cells were transfected with mouse and human WT and mutated netrin-1–AP plasmids and grown for 48 hours. Western blot showed the presence of the WT and mutated proteins in total lysates at the expected molecular weight (A) but no detection of the mutated proteins in the supernatant, contrary to that seen with WT (B). AP assay of the supernatants (C) showed no difference in the initial rate of reaction between nontransfected cells (X) and cells transfected with mutated netrin-1–AP plasmids, indicating that mutated netrin-1–AP levels in the supernatant were under the detection level. The experiments were replicated 3 times. The antibodies used were anti–netrin-1 (A, B, and D–F), anti-actin (A), and anti–α-tubulin (D). Flp-In TRex tetracycline transactivator HeLa cells were transfected with the WT or 3 netrin-1 constructs harboring the NTN1 mutations (D–G). Stable cell lines were grown for 24 hours in the presence or absence of doxycycline. Western blot showed the presence of WT and mutated proteins in total lysates at the expected molecular weight in the presence of doxycycline (D) but no detection of the mutated proteins in the supernatant, contrary to that seen with WT (E). A small amount of mutated proteins could be detected in the supernatant after concentration (F). In all cases, no netrin-1 protein could be detected in the absence of doxycycline (D–F). (G) The ratio of netrin-1/α-tubulin was significantly reduced only for the mutated I518del and C601R forms compared with WT (1-way ANOVA [F (3,12) = 6.44, P = 0.008] followed by Bonferroni’s post-hoc test, *P < 0.05 and **P < 0.01). h, human, m, mouse; Net1, netrin-1. The experiments were replicated 3 times.