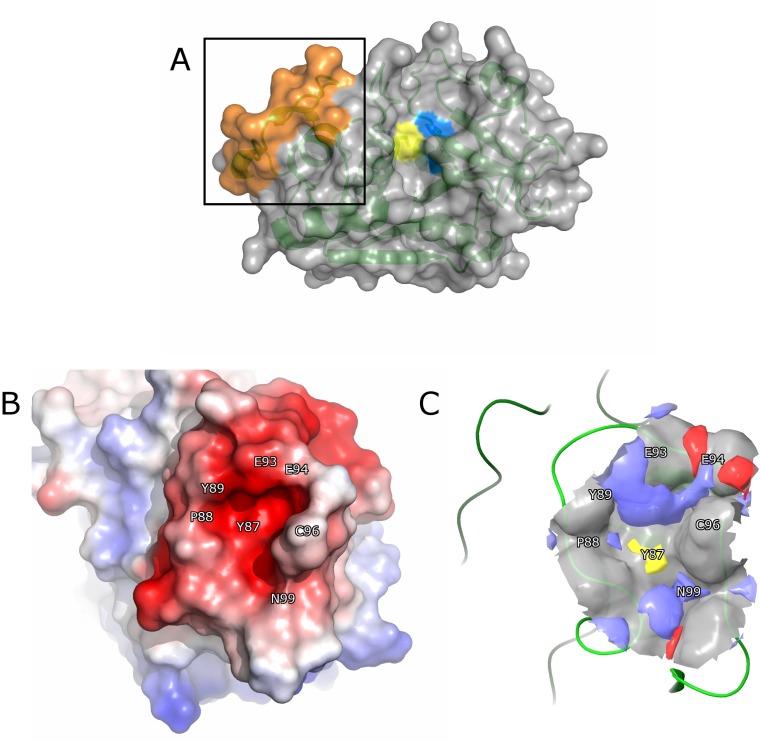
Fig 1. Binding site analysis of ectosteric site 1 in CatK.
(A) Overview of CatK (PDB ID: 1ATK) shown in surface and ribbon form. The active site residues, Cys25 and His162, are colored in yellow and blue, respectively. Ectosteric site 1 is highlighted in the box and colored in orange. (B) The electrostatic potential surface of ectosteric site 1 in CatK displays electronegativity throughout the protein-protein interface as a result of negatively charged residues in this region. (C) The binding pocket in ectosteric site 1 displayed high theoretical druggability (Sitemap, Schrödinger Inc.), (druggability score: 0.816; druggability threshold: 0.80; site score: 0.715) as a result of its favorable geometry and size. Most of the area surrounding the cavity is hydrophilic (red for hydrogen bond-accepting sites, blue for hydrogen bond-donating sites). The potential interacting residues are located surrounding the binding site, Glu93, Glu94, and Gln99 and are shown in blue. The hydrophobic residue, Tyr87, can be seen in the center of the binding site and is shown in yellow.