Figure 1. Study Design of the RADHER trial.
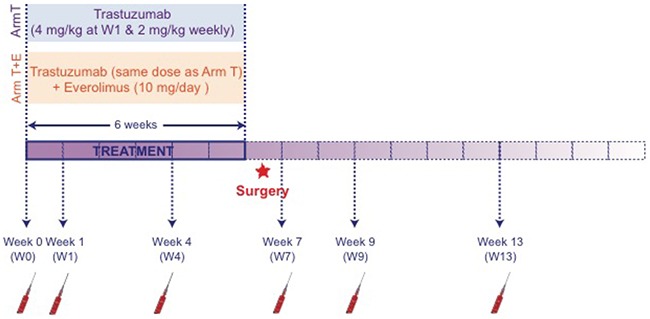
Women patients with early breast cancer (EBC) overexpressing HER-2 were randomized using a 1:1 ratio. Group T was treated with trastuzumab alone; group T+E was administered a combination of trastuzumab and everolimus. Blood samples were collected under fasting conditions, at six different times: at baseline (W0) i.e. before the first therapy cure; one (W1) and four weeks (W4) after the beginning of the treatment; two weeks (W7), four weeks (W9) and eight weeks (W13) after the end of the treatment, i.e. after the last drip of trastuzumab. NMR analysis was performed once the trial completed.
