Abstract
Obesity and diabetes has become a major epidemic across the globe. Controlling obesity has been a challenge since this would require either increased physical activity or reduced caloric intake; both are difficult to enforce. There has been renewed interest in exploiting pathways such as uncoupling protein 1 (UCP1)-mediated uncoupling in brown adipose tissue (BAT) and white adipose tissue to increase energy expenditure to control weight gain. However, relying on UCP1-based thermogenesis alone may not be sufficient to control obesity in humans. On the other hand, skeletal muscle is the largest organ and a major contributor to basal metabolic rate and increasing energy expenditure in muscle through nonshivering thermogenic mechanisms, which can substantially affect whole body metabolism and weight gain. In this review we will describe the role of Sarcolipin-mediated uncoupling of Sarcoplasmic Reticulum Calcium ATPase (SERCA) as a potential mechanism for increased energy expenditure both during cold and diet-induced thermogenesis.
Keywords: ATP hydrolysis, Calcium cycling, Diabetes mellitus, Obesity, Sarcolipin, Sarcoplasmic reticulum calcium-transporting ATPases, Skeletal muscle thermogenesis
INTRODUCTION
Obesity results from an imbalance in caloric intake over energy expenditure and is a major health burden with annual costs exceeding 100 billion dollars [1,2]. The abundance of caloric-rich diets and lack of physical activity has increased the global occurrence of obesity at an alarming rate; equally as alarming is the drastically increasing prevalence of obesity among children [3,4]. The World Health Organization has identified obesity as one of the major emerging chronic diseases of the 21st century. Obesity increases the risk of type 2 diabetes mellitus, hypertension, dyslipidemias, and cardiovascular disease, reducing life expectancy. Thirty-six percent of United States adults are obese and many cannot lose sufficient weight to improve health with lifestyle interventions alone. The currently available weight loss drugs cannot effectively control obesity without serious health side effects. More often than not, physicians recommend regular exercise as the most effective way of controlling weight gain, perhaps second only to dietary caloric restriction. However, it has been extremely difficult to enforce regular exercise to prevent weight gain. A recent study warns of the urgent need for safe and effective strategies to curb the rising prevalence of obesity, and medications may play more prominent role in future therapeutic regimens [5].
Skeletal muscle is the largest organ in the body; in most mammals it makes up to ~45% to 55% body mass and is a major determinant of the basal metabolic rate [6,7,8]. Importantly, muscle can be recruited to increase energy expenditure several fold through physical activity, including sports, voluntary exercise, and/or resistance weight training [9,10,11]. Muscle is responsible for consuming nearly 80% of insulin-stimulated glucose uptake; thus, serving a major role in glucose disposal [9,12]. During prolonged energy demand, muscle has the ability to switch from carbohydrates to fatty acid utilization. In addition to being a contractile machine, skeletal muscle plays a central role in temperature homeostasis; it can be recruited to produce heat through shivering and nonshivering thermogenesis (NST) [13,14]. There is also evidence that skeletal muscle plays an important role in diet-induced thermogenesis [15,16]. Heat production through skeletal muscle shivering is a known mechanism; however, its ability to generate heat through nonshivering mechanisms is not well understood. Studies from our laboratory and others have shown that futile sarcoplasmic reticulum calcium ATPase (SERCA) pump activity induced by sarcolipin (SLN) binding can lead to increased heat production and energy expenditure in muscle (Fig. 1) [17,18,19,20,21]. The primary objective of this review is to highlight recent progress on our understanding of muscle thermogenesis and their role in energy expenditure and whole body metabolism.
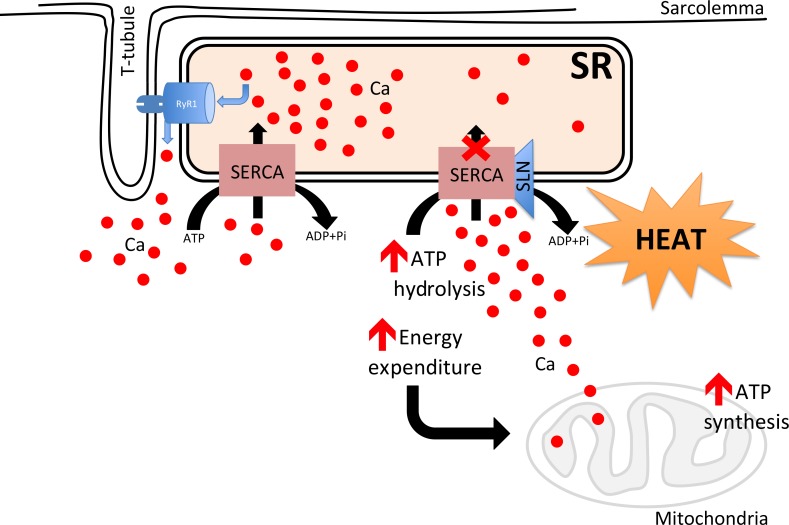
Fig. 1. Proposed mechanism to show how sarcolipin (SLN)/sarcoendoplasmic reticulum calcium APTase (SERCA) interaction affects muscle metabolism. SERCA uses adenosine triphosphate (ATP) hydrolysis to actively transport Ca2+ from the cytosol into the sarcoplasmic reticulum lumen. SLN and Ca2+ bind competitively to SERCA during Ca2+ transport. SLN binding to SERCA does not inhibit ATP hydrolysis but prevents Ca2+ transport by a mechanism named uncoupling, where Ca2+ slips back into cytosol. Uncoupling of SERCA leads to futile cycling of the SERCA pump resulting in increased ATP hydrolysis/heat production; thus, creating energy demand. Uncoupling of SERCA increases cytosolic Ca2+ acutely, thereby promoting Ca2+ entry into mitochondria matrix activating the oxidative metabolism and ATP synthesis. RyR1, ryanodine receptor 1; ADP, adenosine diphosphate; Pi, inorganic phosphate.
MUSCLE IS AN ANCIENT THERMOGENIC ORGAN
Muscle is the primary thermogenic organ in most vertebrates, since muscle contraction is coupled to heat production [22,23]. During muscle contraction, heat is generated through both myosin-mediated adenosine triphosphate (ATP) hydrolysis and Ca2+ transport driven by the SERCA pump. It is well known that heat production in muscle is beneficial, since muscles perform better once warmed up; however, prolonged muscle activity can generate excessive amount of heat. Contraction-mediated heat production is exploited by shivering, a repetitive mode of involuntary contractions resulting in excessive heat production. High intensity shivering activates large muscles and increases glycolysis as the main source for heat production. Since no work is done during shivering, the major part of the chemical energy is liberated as heat within muscle tissue. Constant shivering can be detrimental because it exhausts the muscle and therefore, nonshivering thermogenic mechanisms have evolved to better adapt to colder environments. Many ectoderms have adapted muscle to produce partial endothermy. Among them, fish and reptiles can exploit rhythmic muscle contractions to generate local heat in times of need. To achieve cranial endothermy, for instance, certain types of fish like the opah (Lampris guttatus) have evolved regional endothermy by activating contractions of the extraocular muscles in order to elevate cranial temperatures [24]. Muscle heat production provides selective advantage for the opah: (1) it protects the central nervous system from cold and (2) enhances vision and detection of prey. Among reptiles, brooding pythons can use a type of shivering thermogenesis to keep their eggs warm to promote embryonic development, and then revert to heterothermy after brooding. These examples illustrate that contraction-based heat production has been exploited by vertebrates to achieve partial endothermy long before complete endothermy evolved in birds and mammals.
MUSCLE HEAT PRODUCTION INDEPENDENT OF MUSCLE CONTRACTION: SARCOPLASMIC RETICULUM-Ca2+ CYCLING IS SUFFICIENT TO GENERATE HEAT AND MAINTAIN PARTIAL ENDOTHERMY
In addition to contraction-dependent heat production, certain species of deep sea fishes have evolved a unique mechanism of heat production through a continuous process of Ca2+ release and reuptake [22]. These species of fish include bill fish, sword fish, tuna, and mackerel which have modified muscle to become a heater organ. The heater organ is derived from extra ocular eye muscle which lacks the typical myofibrillar structure but is instead, densely packed with mitochondria and extensive sarcoplasmic reticulum (SR) networks in between [22]. The SR is equipped with both Ca2+ release and reuptake mechanisms found in most muscle. It is believed that neuronal stimulation depolarizes the heater organ sarcolemmal membrane leading to SR-Ca2+ release through the ryanodine receptor (RyR), subsequently activating SR-Ca2+ ATPase to transport the myoplasmic Ca2+ across SR membrane. The translocation of calcium ions from the myoplasm to the SR lumen by SERCA requires ATP hydrolysis; thus, heat is produced by SERCA's ATPase activity without needing muscle contraction. This type of heat production involves futile Ca2+ cycling which can be energetically costly. However, the increased energy demand is well supported by an abundant mitochondrial population in these specialized cells. Of note, fishes have no brown adipose tissue (BAT) so they rely primarily on striated muscle to generate heat. These studies in fish led to the suggestion that muscle can also generate heat independently of muscle contraction through futile SR Ca2+ cycling.
MALIGNANT HYPERTHERMIA AND HEAT PRODUCTION IS DUE TO UNCONTROLLED Ca2+ CYCLING
Skeletal muscle plays an important role in both activity-dependent and independent heat production. Even while an animal is at rest, muscle can contribute to a significant amount of basal energy expenditure and heat production due to its high metabolic activity. Among the mechanisms that contribute to basal heat production is intracellular calcium ion homeostasis driven by SERCA. The SERCA pump depends on ATP hydrolysis for Ca2+ uptake; it translocates 2 Ca2+ ions per ATP molecule hydrolyzed. In resting conditions, the SR luminal concentration of Ca2+ is high (1×10–3M) compared to low cytoplasmic Ca2+ (1×10–7M), which produces a 10,000-fold concentration gradient. Due to this strong gradient, the RyR channel is under high luminal pressure and can spontaneously leak Ca2+, a phenomenon that may be exacerbated by both physiological and pathological changes to RyR protein and or its immediate lipid environment. Malignant hyperthermia (MH), a genetic life threatening disease, is one such example where continuous Ca2+ recycling by the SR membrane can lead to excessive heat production by muscle. MH patients are susceptible to volatile anesthetics (halothane) and often end up with abnormal contracture and hyperthermia [25]. First identified in 1960 in an Australian family who lost 10 members of the family accidently during surgical anesthetics, the disease is found also in pigs, and it is now known that the genetic defect is largely due to mutations in RyR, which encodes the Ca2+ release channel present primarily in skeletal muscle [26,27,28]. The consequence of mutations in the RyR gene is a sustained Ca2+ release following anesthetic exposure; however, the release mechanism itself is not responsible for heat production. A sustained elevation of myoplasmic Ca2+ results in (1) abnormal contracture of muscle due to inability to relax and (2) chronic SERCA pump activity due to continuous Ca2+ leak which results in excessive heat production. Thus, futile Ca2+ cycling by SERCA pump is the primary mechanism for heat production. On the other hand, a sustained level of cytoplasmic Ca2+ activates glycolysis and oxidative metabolism through Ca2+-dependent enzymes, and by Ca2+ itself entering through mitochondria uniporter and acting as a second messenger to activate key enzymes (pyruvate dehydrogenase, ATP synthase). Without pharmacological intervention this can lead to both excessive heat production and energy demand resulting in a hypermetabolic state. Since the development of the drug named dantrolene, an inhibitor of RyR, MH has been tightly controlled [29]. Studies in MH in both pigs and human have highlighted that SR Ca2+ cycling can be an important mechanism for heat production and energy expenditure. However, its contribution to the latter is often overlooked by muscle physiologists due to the belief that it is a component of contractile activity of the muscle.
SKELETAL MUSCLE, FIBER TYPES, AND THEIR ROLE IN THERMOGENESIS AND METABOLISM
Skeletal muscle is highly dependent on energy supply, and its energy demand varies by several orders of magnitude depending on its needs (active or at rest), and depending on the fiber type. Thus, energy metabolism has to be tightly regulated in order to meet varying energy requirements [30]. Mammalian skeletal muscle was initially characterized into fast and slow fiber types, based on their contractile characteristics that are determined by myosin isoforms IIb, IIa, IIx, and type I. But the skeletal muscle has also been classified into three different fiber types based on their metabolic characteristics, as follows.
Slow oxidative fibers
These fibers are found in muscle groups that are responsible for posture maintenance such as soleus muscle. The slow twitch muscle expresses type I myosin isoform, SERCA2a, and SLN. It is red in color due to high vascularization, is quite rich in mitochondria and predominantly relies on fatty acid as a substrate to meet the high-energy demands of the postural muscles. These muscles contract slowly, produce lesser force but are more resistant to fatigue, and are recruited during prolonged muscle activity such as marathon running and adaptation to cold.
Fast oxidative fibers
These fibers are abundant in most muscle groups in large mammals. These fibers express type IIA myosin isoform, SERCA1a, and SLN. They have an intermediate diameter, capillary volume and mitochondrial density. These fibers can generate explosive power for a short period and are often recruited during short burst of activities such as basketball, soccer, tennis, and hockey. They rely on both glycolysis and oxidative metabolism and have the ability to switch between carbohydrates and fatty acid utilization depending on substrate availability. These muscle fibers produce more force but they exhibit less fatigue resistance, so they are in the middle of the muscle fiber spectrum.
Fast glycolytic fibers
These muscle fibers are the fastest and they can generate the most power and speed, and they are present in large muscle groups including gastrocnemius and quadriceps. They express type 2b and 2x myosin isoforms, SERCA1a but do not contain SLN. These types of fibers are recruited in activities that require an all-out burst of power and only act for an extremely short period of time, as the total length of their contractions usually last ~7.5 milliseconds. They tend to fatigue very quickly compared to fast oxidative fibers because they rely primarily on anaerobic glycolysis to produce ATP, a process in which lactic acid accumulates and promotes a condition called acidosis, compromising muscle function.
All three types of muscle fibers are recruited during shivering-induced thermogenesis, although some studies have suggested that glycolytic fibers are most recruited during high intensity shivering in rodents and oxidative fibers during low intensity shivering. During prolonged cold adaptation shivering is completely replaced by NST and the mechanism behind NST is just only beginning to be understood.
MUSCLE IS A SITE OF SHIVERING AND NONSHIVERING THERMOGENESIS
Shivering is the most well-known form of heat production in muscle and it is activated during an acute cold exposure. Only birds and mammals have the ability to shiver, and the mechanism is similar to muscle contraction. During shivering, heat is primarily produced by the major ATP-utilizing enzymes, notably, myosin ATPase and SERCA. Although shivering is the first response to an acute cold exposure, shivering is energetically very costly and may even compromise muscle function. In addition to shivering, mammals have evolved NST mechanisms to adapt and thrive in colder climates. Pioneering studies performed in newborn mammals and rodents have shown that BAT is an important site of NST [31,32,33]. BAT is a highly specialized organ enriched with mitochondria that expresses a mitochondrial transmembrane protein called uncoupling protein 1 (UCP1) [31,32,33,34]. Significant progress has been made towards understanding the activation and regulation of UCP1-dependent thermogenesis in BAT [35,36,37]. However, in large mammals including humans, BAT is restricted to neonatal stages and becomes a minor component in adult life. Therefore, large mammals especially humans have to rely on muscle-based thermogenesis for temperature homeostasis [23]. Interestingly, BAT is either absent or inactive in certain endotherms, particularly in birds. In some mammals (boars and pigs), the gene encoding UCP1 is mutated and they must completely rely on muscle for thermogenesis [23]. These findings suggested that there must be other NST which may also contribute to whole body temperature (Tc).
The first evidence that skeletal muscle can also be an important site of NST comes from studies performed in avian species by Duchamp et al. [38,39]. Using direct blood-flow measurements in 5-week thermoneutral- (TN, 25℃) and cold-acclimatized (CA, 4℃) ducklings (Cairina moschata), and then exposed to 8℃, they were able to show that total muscle blood flow increased equally in the TN and CA ducklings, but the CA ducklings did not shiver compared to TN. Both groups were also able to maintain Tc in the optimal range [38,39]. Thus, skeletal muscles from CA ducklings were able to produce the same amount of heat as muscles from shivering TN ducklings, demonstrating the existence of NST in skeletal muscle. Although these studies identified that muscle could serve as a site of NST, they did not provide a detailed mechanism for the source of heat production. In a subsequent study, Dumonteil et al. [40] performed detailed analyses to show how changes in SERCA and RyR gene expression patterns coincided with the activation of NST. Thus, these studies indicated that enhanced Ca2+ cycling could be responsible for muscle-based NST. Studies conducted by Arruda et al. [41,42] also showed that when rabbits are CA, SERCA1 expression is increased in red muscle (SERCA2 levels are unaffected) but not in white muscle. Furthermore, in vitro preparations of these CA muscles showed that cold exposure increased the heat released during ATP hydrolysis 2-fold in red muscle, in which oxidative (mitochondrial) capacity is at least 2-fold greater than white muscle. Thus, cold exposure increased the heat-generating capacity of rabbit red muscle [43]. These results also suggest that enhanced Ca2+ cycling might be involved in heat production.
THE ROLE OF SLN AND SERCA PUMP IN MUSCLE NONSHIVERING THERMOGENESIS
The SERCA pump plays a central role in SR Ca2+ cycling and muscle contraction. By actively transporting Ca2+, it maintains a low cytosolic but a high luminal SR Ca2+ concentration. The SERCA pump in skeletal muscle is encoded by SERCA1 and SERCA2 genes; SERCA1a being the major isoform in fast twitch fibers, whereas the slow twitch/oxidative fibers express both SERCA1a and SERCA2a protein [14,44,45]. SERCA activity is regulated by two small molecular weight proteins in muscle, namely phospholamban (PLB, 52 aa) and SLN (31 aa) [17,19,46]. Although PLB and SLN occupy the same site on SERCA protein, they affect SERCA pump activity very differently. PLB binding inhibits SERCA pump activity (at low cytosolic Ca2+) and its inhibitory interaction is relieved by an increase in cytosolic Ca2+ and or phosphorylation of PLB. The mechanism of PLB interaction and regulation of SERCA pump activity has been extensively reviewed and therefore will not be discussed here. SLN is encoded by a single gene, composed of two exons and differs structurally from PLB. SLN expression is predominant in skeletal muscle but is also present in atrial chamber of the heart [46]. SLN protein expression is high in embryonic/neonatal skeletal muscle; whereas in adult stages it is absent in glycolytic muscle fibers but is expressed abundantly in oxidative and slow twitch muscle fibers. In comparison to rodents, SLN expression is several folds higher in large mammals (including human) due to the high proportion of oxidative/slow twitch fibers [13,23].
Although SLN was discovered nearly 30 years ago, its exact function remained largely unknown. In an effort to understand its role in muscle, we began studying how SLN binding affects SERCA activity using an in vitro biochemical approach. Our biochemical studies showed that SLN binds to SERCA even at high cytoplasmic Ca2+ and remains bound to SERCA during the Ca2+ transport cycle. In contrast, PLB binding to SERCA only occurs in the Ca2+-free state; therefore, Ca2+ and PLB binding to SERCA are mutually exclusive. Interestingly, SLN binding to SERCA does not affect ATP hydrolysis, but decreases the Vmax of Ca2+ uptake by blocking Ca2+ transport into the SR lumen. Using reconstituted synthetic SLN and SERCA, Mall et al. [47] and Smith et al. [48] initially proposed the idea that SLN binding to SERCA could promote uncoupling of ATP hydrolysis from Ca2+ transport. They showed that Ca2+ accumulation in the vesicles decreased, but the heat released by SERCA increased in the presence of SLN. These studies suggested that SLN binding to SERCA promotes slippage of Ca2+ back into the cytosol and the energy from the resulting ATP hydrolysis would thus be released as heat, without any Ca2+ transport. These and other studies suggested that binding of SLN promotes futile cycling of SERCA pump, resulting in a total increase in ATP hydrolysis and heat production.
SLN PLAYS A KEY ROLE IN COLD-INDUCED MUSCLE THERMOGENESIS
Although initial in vitro studies predicted that SLN could play a role in muscle thermogenesis, direct evidence in support of this was missing. Therefore, our laboratory generated SLN knockout mice (SLN-KO) and explored how SLN impacts muscle function and muscle-based thermogenesis [20,21]. Interestingly, loss of SLN did not affect muscle growth and/or function and the KO mice could not be distinguished easily from its wild type (WT) littermates. Regarding muscle-based thermogenesis, we decided to challenge the SLN-KO mice to acute cold (4℃) in a temperature controlled Comprehensive Lab Animal Monitoring System (CLAMS) set up [20]. We surgically removed interscapular BAT (iBAT) to minimize contribution from BAT, a key contributor to thermogenesis. Surprisingly, we found that iBAT-ablated SLN-KO mice were unable to maintain their Tc; within the first 4 hours, the majority developed hypothermia, and would die if not removed from cold [20]. On the other hand, WT (iBAT-ablated control mice) were able to maintain their body Tc despite having SLN expression restricted mainly to oxidative fibers that are non-abundant in mice, what highlights the importance of SLN in muscle thermogenesis. This was further confirmed by the finding that SLN-KO mice could be rescued following reintroduction of SLN [20,49]. These in vivo studies using SLN null mice validated the initial in vitro findings, which suggested that SLN interaction with SERCA promotes uncoupling of SERCA, resulting in increased ATP hydrolysis and heat production.
Unlike large mammals, rodents contain substantial BAT in their adult life and BAT is very important for cold adaptation in rodents. Interestingly, studies in UCP1-KO mice showed that while these mice are cold sensitive, they can be gradually cold adapted to 4℃, suggesting compensation by muscle-based thermogenic mechanisms [50]. Therefore, we examined if there was a cross-talk between muscle and BAT-dependent thermogenesis during cold adaptation and if they could compensate for each other when one mechanism would fail. Our studies revealed that cold adaptation in mice relies on both muscle and BAT-based thermogenesis. Interestingly, even mild cold exposure recruits both muscle and BAT-based heat production [51]. We next investigated if muscle could compensate for the loss of BAT function using either iBAT-ablated or UCP1-KO. We found that gradual cold adaptation of iBAT ablated or UCP1-KO mice upregulates SLN expression in the skeletal muscle; which suggests that muscle thermogenesis is recruited to a greater extent when BAT function is minimized [51,52,53]. We have also shown that when iBAT was surgically removed, the mice were able to adapt to cold but at an increased energy cost [51]. The skeletal muscles in these mice underwent extensive remodeling of both SR and mitochondria, including alteration in the expression of key components of Ca2+ handling such as SLN, SERCA, and RyR1. Interestingly, when neonatal mice were cold-adapted to 4℃, the normally occurring developmental SLN downregulation in fast twitch muscle was prevented. These studies further showed that SLN/SERCA-based thermogenesis could be an important NST mechanism in skeletal muscle.
SLN PLAYS AN IMPORTANT ROLE IN DIET-INDUCED THERMOGENESIS IN MUSCLE
The concept of diet induced thermogenesis (DIT) was first described by Rothwell and Stock [54] and Stock [55]. They showed that rodents can increase energy expenditure in response to overfeeding to prevent excessive weight gain. They suggested that (DIT) can be beneficial to dispose excess calories and prevent metabolic diseases. The role of BAT and UCP1 has been extensively studied as a major mechanism for DIT in rodents [33,36,37,55,56,57]. Although the detailed mechanism responsible for DIT remains less well understood, there is substantial evidence to show that diet rich in fat activates heat production in BAT due to increased sympathetic nervous system activity [58]. Since muscle is a consumer of metabolites and SLN uncoupling of SERCA can increase energy expenditure, we wanted to further explore the role of SLN in diet-induced thermogenesis in muscle. We initially showed that SLN-KO mice were prone to diet-induced obesity when fed with high fat diet (HFD); whereas WT mice showed increased SLN expression [20]. To understand the relative contribution of BAT versus skeletal muscle to DIT, UCP1-KO, SLN-KO, and SLN overexpression (SLN-OE) mice were fed with a HFD for a period of 12 weeks [59,60]. A key finding was that SLN-KO mice gained comparable weight as UCP1-KO mice on HFD, suggesting that loss of muscle-based thermogenesis has similar consequences on weight gain as loss of BAT-mediated DIT [60]. This may suggest that both SLN- and UCP1-based thermogenesis contribute to DIT to a similar extent in rodents. Although SLN-KO mice had intact BAT, SLN deficiency was sufficient to cause increased obesity, which suggests that muscle-based NST is a critical component of DIT and can be very well recruited during caloric excess. We further investigated if SLN-OE in both glycolytic and oxidative muscles can be beneficial to increase fatty acid oxidative metabolism. We found that mice overexpressing SLN were significantly less obese than WT mice and were resistant to HFD-induced obesity [59]. An interesting finding was that fast glycolytic skeletal muscles such as tibialis anterior and extensor digitorum longus from SLN-OE mice showed a striking increase in mitochondrial content and upregulation of metabolic enzymes involved in fatty acid oxidation, suggesting that SLN promotes oxidative metabolism. Considering that muscle represents ~45% to 50% body mass, even small increases in the energy demand/expenditure in muscle can have significant effects on whole-body energy expenditure [15,16]. These studies led us to suggest that SLN expression can create both energy demand and increase energy expenditure through increased oxidative metabolism and can be a novel mechanism to increase energy expenditure in muscle.
CONCLUSIONS
Obesity and diabetes have increased globally during the last two decades at an alarming rate. Many factors have contributed to the growth and prevalence of obesity. These include excessive consumption of caloric-rich diet, limited physical activity and urban life style. There are no effective treatments to reduce obesity other than caloric restriction and exercise which are difficult to enforce on a daily basis. Our hope is to look for answers within our own body and, skeletal muscle has enormous potential due to its ability to increase metabolism significantly and may be our best choice to increase energy expenditure. As a result of life style change, there is a significant reduction in physical activity, and so we have to find alternate ways to promote energy expenditure and even if it entails taking pills to enhance this process. The discovery of SLN as an uncoupler of SERCA pump provides a promising target to increase energy expenditure in muscle. The molecular basis behind NST requires further investigation towards the identification of upstream mechanisms and pathways from SLN to induce muscle NST. It is our hope that future research will endeavor to identify novel targets to pharmacologically activate energy expenditure in muscle, since this will become critical in conditions where physical activity is increasingly limited. One of the directions worth exploring is the synergistic effect of both cold and pharmacological agents known to increase energy expenditure in muscle, in a combined therapy.
ACKNOWLEDGMENTS
We thank all the previous members of Dr. Periasamy laboratory who have contributed to the original studies cited in this review manuscript. This work was supported in part, by National Institutes of Health Grants R01-HL 088555 and R01 DK098240 and a basic Science grant from ADA 7-13-BS-131 to Muthu Periasamy.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 2.Lepor NE, Fouchia DD, McCullough PA. New vistas for the treatment of obesity: turning the tide against the leading cause of morbidity and cardiovascular mortality in the developed world. Rev Cardiovasc Med. 2013;14:20–39. doi: 10.3909/ricm0682. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, Flegal KM. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA. 2016;315:2292–2299. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011-2014. NCHS Data Brief. 2015;(219):1–8. [PubMed] [Google Scholar]
- 5.Padwal R, Li SK, Lau DC. Long-term pharmacotherapy for overweight and obesity: a systematic review and meta-analysis of randomized controlled trials. Int J Obes Relat Metab Disord. 2003;27:1437–1446. doi: 10.1038/sj.ijo.0802475. [DOI] [PubMed] [Google Scholar]
- 6.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (1985) 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 7.Zurlo F, Nemeth PM, Choksi RM, Sesodia S, Ravussin E. Whole-body energy metabolism and skeletal muscle biochemical characteristics. Metabolism. 1994;43:481–486. doi: 10.1016/0026-0495(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 8.Zurlo F, Larson K, Bogardus C, Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest. 1990;86:1423–1427. doi: 10.1172/JCI114857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrannini E, Simonson DC, Katz LD, Reichard G, Jr, Bevilacqua S, Barrett EJ, Olsson M, DeFronzo RA. The disposal of an oral glucose load in patients with non-insulin-dependent diabetes. Metabolism. 1988;37:79–85. doi: 10.1016/0026-0495(88)90033-9. [DOI] [PubMed] [Google Scholar]
- 10.Kelley DE. Skeletal muscle fat oxidation: timing and flexibility are everything. J Clin Invest. 2005;115:1699–1702. doi: 10.1172/JCI25758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner N, Cooney GJ, Kraegen EW, Bruce CR. Fatty acid metabolism, energy expenditure and insulin resistance in muscle. J Endocrinol. 2014;220:T61–T79. doi: 10.1530/JOE-13-0397. [DOI] [PubMed] [Google Scholar]
- 12.Thiebaud D, Jacot E, DeFronzo RA, Maeder E, Jequier E, Felber JP. The effect of graded doses of insulin on total glucose uptake, glucose oxidation, and glucose storage in man. Diabetes. 1982;31:957–963. doi: 10.2337/diacare.31.11.957. [DOI] [PubMed] [Google Scholar]
- 13.Pant M, Bal NC, Periasamy M. Sarcolipin: a key thermogenic and metabolic regulator in skeletal muscle. Trends Endocrinol Metab. 2016;27:881–892. doi: 10.1016/j.tem.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Periasamy M, Maurya SK, Sahoo SK, Singh S, Sahoo SK, Reis FCG, Bal NC. Role of SERCA pump in muscle thermogenesis and metabolism. Compr Physiol. 2017;7:879–890. doi: 10.1002/cphy.c160030. [DOI] [PubMed] [Google Scholar]
- 15.Lowell BB, Bachman ES. Beta-adrenergic receptors, diet-induced thermogenesis, and obesity. J Biol Chem. 2003;278:29385–29388. doi: 10.1074/jbc.R300011200. [DOI] [PubMed] [Google Scholar]
- 16.Maurya SK, Periasamy M. Sarcolipin is a novel regulator of muscle metabolism and obesity. Pharmacol Res. 2015;102:270–275. doi: 10.1016/j.phrs.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahoo SK, Shaikh SA, Sopariwala DH, Bal NC, Periasamy M. Sarcolipin protein interaction with sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) is distinct from phospholamban protein, and only sarcolipin can promote uncoupling of the SERCA pump. J Biol Chem. 2013;288:6881–6889. doi: 10.1074/jbc.M112.436915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahoo SK, Shaikh SA, Sopariwala DH, Bal NC, Bruhn DS, Kopec W, Khandelia H, Periasamy M. The N terminus of sarcolipin plays an important role in uncoupling sarco-endoplasmic reticulum Ca2+-ATPase (SERCA) ATP hydrolysis from Ca2+ transport. J Biol Chem. 2015;290:14057–14067. doi: 10.1074/jbc.M115.636738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaikh SA, Sahoo SK, Periasamy M. Phospholamban and sarcolipin: are they functionally redundant or distinct regulators of the sarco(endo)plasmic reticulum calcium ATPase? J Mol Cell Cardiol. 2016;91:81–91. doi: 10.1016/j.yjmcc.2015.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bal NC, Maurya SK, Sopariwala DH, Sahoo SK, Gupta SC, Shaikh SA, Pant M, Rowland LA, Bombardier E, Goonasekera SA, Tupling AR, Molkentin JD, Periasamy M. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat Med. 2012;18:1575–1579. doi: 10.1038/nm.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babu GJ, Bhupathy P, Timofeyev V, Petrashevskaya NN, Reiser PJ, Chiamvimonvat N, Periasamy M. Ablation of sarcolipin enhances sarcoplasmic reticulum calcium transport and atrial contractility. Proc Natl Acad Sci U S A. 2007;104:17867–17872. doi: 10.1073/pnas.0707722104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Block BA. Thermogenesis in muscle. Annu Rev Physiol. 1994;56:535–577. doi: 10.1146/annurev.ph.56.030194.002535. [DOI] [PubMed] [Google Scholar]
- 23.Rowland LA, Bal NC, Periasamy M. The role of skeletal-muscle-based thermogenic mechanisms in vertebrate endothermy. Biol Rev Camb Philos Soc. 2015;90:1279–1297. doi: 10.1111/brv.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Runcie RM, Dewar H, Hawn DR, Frank LR, Dickson KA. Evidence for cranial endothermy in the opah (Lampris guttatus) J Exp Biol. 2009;212(Pt 4):461–470. doi: 10.1242/jeb.022814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg H, Pollock N, Schiemann A, Bulger T, Stowell K. Malignant hyperthermia: a review. Orphanet J Rare Dis. 2015;10:93. doi: 10.1186/s13023-015-0310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacLennan DH. The genetic basis of malignant hyperthermia. Trends Pharmacol Sci. 1992;13:330–334. doi: 10.1016/0165-6147(92)90101-b. [DOI] [PubMed] [Google Scholar]
- 27.MacLennan DH, Otsu K, Fujii J, Zorzato F, Phillips MS, O'Brien PJ, Archibald AL, Britt BA, Gillard EF, Worton RG. The role of the skeletal muscle ryanodine receptor gene in malignant hyperthermia. Symp Soc Exp Biol. 1992;46:189–201. [PubMed] [Google Scholar]
- 28.MacLennan DH, Phillips MS. Malignant hyperthermia. Science. 1992;256:789–794. doi: 10.1126/science.1589759. [DOI] [PubMed] [Google Scholar]
- 29.Sharma A, Karnik H, Kukreja S, Jagger K. Malignant hyperthermia: dantrolene sodium. A must have. Indian J Anaesth. 2012;56:212–213. doi: 10.4103/0019-5049.96327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Das AM, Steuerwald U, Illsinger S. Inborn errors of energy metabolism associated with myopathies. J Biomed Biotechnol. 2010;2010:340849. doi: 10.1155/2010/340849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cannon B, Houstek J, Nedergaard J. Brown adipose tissue. More than an effector of thermogenesis? Ann N Y Acad Sci. 1998;856:171–187. doi: 10.1111/j.1749-6632.1998.tb08325.x. [DOI] [PubMed] [Google Scholar]
- 32.Cannon B, Jacobsson A, Rehnmark S, Nedergaard J. Signal transduction in brown adipose tissue recruitment: noradrenaline and beyond. Int J Obes Relat Metab Disord. 1996;20(Suppl 3):S36–S42. [PubMed] [Google Scholar]
- 33.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 34.Kozak LP, Harper ME. Mitochondrial uncoupling proteins in energy expenditure. Annu Rev Nutr. 2000;20:339–363. doi: 10.1146/annurev.nutr.20.1.339. [DOI] [PubMed] [Google Scholar]
- 35.Meyer CW, Willershauser M, Jastroch M, Rourke BC, Fromme T, Oelkrug R, Heldmaier G, Klingenspor M. Adaptive thermogenesis and thermal conductance in wild-type and UCP1-KO mice. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1396–R1406. doi: 10.1152/ajpregu.00021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kozak LP, Anunciado-Koza R. UCP1: its involvement and utility in obesity. Int J Obes (Lond) 2008;32(Suppl 7):S32–S38. doi: 10.1038/ijo.2008.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kozak LP, Koza RA. Mitochondria uncoupling proteins and obesity: molecular and genetic aspects of UCP1. Int J Obes Relat Metab Disord. 1999;23(Suppl 6):S33–S37. doi: 10.1038/sj.ijo.0800941. [DOI] [PubMed] [Google Scholar]
- 38.Duchamp C, Barre H. Skeletal muscle as the major site of nonshivering thermogenesis in cold-acclimated ducklings. Am J Physiol. 1993;265(5 Pt 2):R1076–R1083. doi: 10.1152/ajpregu.1993.265.5.R1076. [DOI] [PubMed] [Google Scholar]
- 39.Duchamp C, Cohen-Adad F, Rouanet JL, Barre H. Histochemical arguments for muscular non-shivering thermogenesis in muscovy ducklings. J Physiol. 1992;457:27–45. doi: 10.1113/jphysiol.1992.sp019363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dumonteil E, Barre H, Meissner G. Sarcoplasmic reticulum Ca(2+)-ATPase and ryanodine receptor in cold-acclimated ducklings and thermogenesis. Am J Physiol. 1993;265(2 Pt 1):C507–C513. doi: 10.1152/ajpcell.1993.265.2.C507. [DOI] [PubMed] [Google Scholar]
- 41.Arruda AP, Nigro M, Oliveira GM, de Meis L. Thermogenic activity of Ca2+-ATPase from skeletal muscle heavy sarcoplasmic reticulum: the role of ryanodine Ca2+ channel. Biochim Biophys Acta. 2007;1768:1498–1505. doi: 10.1016/j.bbamem.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 42.Arruda AP, Ketzer LA, Nigro M, Galina A, Carvalho DP, de Meis L. Cold tolerance in hypothyroid rabbits: role of skeletal muscle mitochondria and sarcoplasmic reticulum Ca2+ ATPase isoform 1 heat production. Endocrinology. 2008;149:6262–6271. doi: 10.1210/en.2008-0564. [DOI] [PubMed] [Google Scholar]
- 43.de Meis L, Arruda AP, Carvalho DP. Role of sarco/endoplasmic reticulum Ca(2+)-ATPase in thermogenesis. Biosci Rep. 2005;25:181–190. doi: 10.1007/s10540-005-2884-7. [DOI] [PubMed] [Google Scholar]
- 44.Periasamy M, Kalyanasundaram A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve. 2007;35:430–442. doi: 10.1002/mus.20745. [DOI] [PubMed] [Google Scholar]
- 45.Periasamy M, Huke S. SERCA pump level is a critical determinant of Ca(2+)homeostasis and cardiac contractility. J Mol Cell Cardiol. 2001;33:1053–1063. doi: 10.1006/jmcc.2001.1366. [DOI] [PubMed] [Google Scholar]
- 46.Babu GJ, Bhupathy P, Carnes CA, Billman GE, Periasamy M. Differential expression of sarcolipin protein during muscle development and cardiac pathophysiology. J Mol Cell Cardiol. 2007;43:215–222. doi: 10.1016/j.yjmcc.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mall S, Broadbridge R, Harrison SL, Gore MG, Lee AG, East JM. The presence of sarcolipin results in increased heat production by Ca(2+)-ATPase. J Biol Chem. 2006;281:36597–36602. doi: 10.1074/jbc.M606869200. [DOI] [PubMed] [Google Scholar]
- 48.Smith WS, Broadbridge R, East JM, Lee AG. Sarcolipin uncouples hydrolysis of ATP from accumulation of Ca2+ by the Ca2+-ATPase of skeletal-muscle sarcoplasmic reticulum. Biochem J. 2002;361(Pt 2):277–286. doi: 10.1042/0264-6021:3610277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sopariwala DH, Pant M, Shaikh SA, Goonasekera SA, Molkentin JD, Weisleder N, Ma J, Pan Z, Periasamy M. Sarcolipin overexpression improves muscle energetics and reduces fatigue. J Appl Physiol (1985) 2015;118:1050–1058. doi: 10.1152/japplphysiol.01066.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ukropec J, Anunciado RP, Ravussin Y, Hulver MW, Kozak LP. UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1-/- mice. J Biol Chem. 2006;281:31894–31908. doi: 10.1074/jbc.M606114200. [DOI] [PubMed] [Google Scholar]
- 51.Bal NC, Maurya SK, Singh S, Wehrens XH, Periasamy M. Increased reliance on muscle-based thermogenesis upon acute minimization of brown adipose tissue function. J Biol Chem. 2016;291:17247–17257. doi: 10.1074/jbc.M116.728188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rowland LA, Bal NC, Kozak LP, Periasamy M. Uncoupling protein 1 and sarcolipin are required to maintain optimal thermogenesis, and loss of both systems compromises survival of mice under cold stress. J Biol Chem. 2015;290:12282–12289. doi: 10.1074/jbc.M115.637603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bal NC, Singh S, Reis FCG, Maurya SK, Pani S, Rowland LA, Periasamy M. Both brown adipose tissue and skeletal muscle thermogenesis processes are activated during mild to severe cold adaptation in mice. J Biol Chem. 2017;292:16616–16625. doi: 10.1074/jbc.M117.790451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rothwell NJ, Stock MJ. Diet-induced thermogenesis. Adv Nutr Res. 1983;5:201–220. doi: 10.1007/978-1-4613-9937-7_9. [DOI] [PubMed] [Google Scholar]
- 55.Stock MJ. The role of brown adipose tissue in diet-induced thermogenesis. Proc Nutr Soc. 1989;48:189–196. doi: 10.1079/pns19890029. [DOI] [PubMed] [Google Scholar]
- 56.Kozak LP. Brown fat and the myth of diet-induced thermogenesis. Cell Metab. 2010;11:263–267. doi: 10.1016/j.cmet.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kozak LP, Koza RA, Anunciado-Koza R. Brown fat thermogenesis and body weight regulation in mice: relevance to humans. Int J Obes (Lond) 2010;34(Suppl 1):S23–S27. doi: 10.1038/ijo.2010.179. [DOI] [PubMed] [Google Scholar]
- 58.Rothwell NJ, Stock MJ. Sympathetic and adrenocorticoid influences on diet-induced thermogenesis and brown fat activity in the rat. Comp Biochem Physiol A Comp Physiol. 1984;79:575–579. doi: 10.1016/0300-9629(84)90451-1. [DOI] [PubMed] [Google Scholar]
- 59.Maurya SK, Bal NC, Sopariwala DH, Pant M, Rowland LA, Shaikh SA, Periasamy M. Sarcolipin is a key determinant of the basal metabolic rate, and its overexpression enhances energy expenditure and resistance against diet-induced obesity. J Biol Chem. 2015;290:10840–10849. doi: 10.1074/jbc.M115.636878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rowland LA, Maurya SK, Bal NC, Kozak L, Periasamy M. Sarcolipin and uncoupling protein 1 play distinct roles in diet-induced thermogenesis and do not compensate for one another. Obesity (Silver Spring) 2016;24:1430–1433. doi: 10.1002/oby.21542. [DOI] [PMC free article] [PubMed] [Google Scholar]