Abstract
An ultimate goal in carbon nanoscience is to decipher formation mechanisms of highly ordered systems. Here, we disclose chemical processes that result in formation of high-symmetry clusterfullerenes, which attract interest for use in applications that span biomedicine to molecular electronics. The conversion of doped graphite into a C80 cage is shown to occur through bottom-up self-assembly reactions. Unlike conventional forms of fullerene, the iconic Buckminsterfullerene cage, I h-C60, is entirely avoided in the bottom-up formation mechanism to afford synthesis of group 3-based metallic nitride clusterfullerenes. The effects of structural motifs and cluster–cage interactions on formation of compounds in the solvent-extractable C70–C100 region are determined by in situ studies of defined clusterfullerenes under typical synthetic conditions. This work establishes the molecular origin and mechanism that underlie formation of unique carbon cage materials, which may be used as a benchmark to guide future nanocarbon explorations.
An understanding of how caged carbon materials self-assemble from doped graphite is a long-standing challenge. Here, the authors show that distinct bottom-up processes lead to the synthesis of high-symmetry clusterfullerenes.
Introduction
Fullerenes that encapsulate clusters of atoms represent a fundamental interest in chemistry, materials, and carbon science due to their unique properties and nanoscale structures1–3. Compounds that entrap the trimetallic nitride cluster are among the most intensively studied form of molecular nanocarbon because they offer promise as contrast agents and other biomedical diagnostics, photovoltaics, and molecular electronics4–7. In particular, Sc3N@I h-C80 mysteriously forms as the “third most abundant fullerene”, only empty C60 and C70 have been isolated in higher yield8, 9. The M3N (M = metal) cluster imparts stability to cage sizes from ~C70 to C100 and donates six electrons to the carbon cage10, 11. Nitride clusterfullerene (NCF) compounds possess diverse structural motifs that are relevant to other carbon networks, such as nanotubes and graphene. For example, non-isolated pentagon rule (non-IPR), Sc3N@D 3-C68, as well as an isomer of the C66 cage exhibit multiple configurations of fused pentagons12–14. Very recently, a heptagon-containing structure was characterized as the NCF, Sc2LaN@C80(hept)15.
An understanding of how these compounds form by simple vaporization of doped graphite is paramount because the intrinsic mechanisms and chemical principles that control formation may be exploited to create entirely new forms of nanomaterials and overcome obstructions in synthesis of cluster-encapsulated carbon materials. Recently, top-down proposals have been rationally inferred as a possible route to formation of the archetypal NCF, M3N@I h-C80, and other high-symmetry fullerenes based on molecular evidence, computational studies, and observations of graphene under electron beam irradiation16–19. In this case, carbon sheets are envisioned to warp into giant cages and subsequently shrink into the icosahedral C80 cage. However, it remains unknown how an icosahedral cage that entraps a four-atom cluster may self-assemble from graphite vaporization, because in situ studies are not possible by conventional synthesis methods. Such in situ studies are a challenging endeavor, but are crucial by virtue that carbon is an extraordinary element that possesses the tendency to exhibit different chemistry under conditions that diverge from characteristic synthesis due to its versatile bonding properties. Interestingly, the conversion of polycyclic aromatic hydrocarbons or their derivatives to fullerenes shares some conceptual similarity to those proposed top-down mechanisms20.
Here, we show that clusterfullerenes are formed from doped graphite, a universal starting material for carbon nanostructure synthesis, by a laser-based synthesis method that permits in situ formation investigations and uncover unprecedented mechanistic insight into self-assembly of complex carbon compounds. We disclose that the formation of high-symmetry clusterfullerenes occurs through a distinct bottom-up mechanism and that high-yield formation of Sc3N@C80 is achieved by the complete circumvention of Buckminsterfullerene, C60 21, in bottom-up reaction paths. To validate our model and probe cage selection and cluster size effects, we directly study the small, non-IPR compound, Sc3N@D 3-C68, and larger, high-symmetry species, including M3N@I h-C80 (M = group 3 metal), under precise synthetic conditions.
Results
M3N-encapsulated (M = group 3) cages from doped graphite
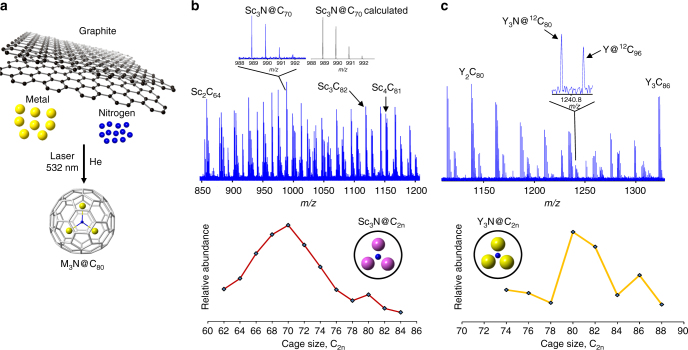
To devise a strategy to study in situ self-assembly processes for these nanomaterials, we performed extensive analyses by laser vaporization of graphite-based starting materials doped with group 3 metal oxides and numerous sources of heteroatoms, such as gaseous (e.g., ammonia and N2) and molecular nitrogen sources. In fact, these heteroatom sources are used in arc discharge synthesis to produce macroscopic quantities of the compounds22. In our approach, online chemical sampling is carried out by use of a pulsed laser vaporization cluster source, analyzed by state-of-the-art Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometry, which was previously limited to empty cages and conventional metallofullerenes23–25. We find that the solid organic nitrogen sources26 are an excellent choice for the formation of trimetallic NCFs under the present laser-based conditions and, in particular, melamine yields highly reproducible formation products27, 28. In these strongly ionizing environments, positive ions are expected to be representative of the neutral NCF distributions, similar to empty cages and mono-metallofullerenes23. Figure 1 shows molecular nanocarbon products formed by laser vaporization (532 nm, 10 mJ per pulse) of a stationary target rod comprised of graphite, scandium oxide, and melamine (10% atom Sc, 1:2 ratio for Sc:C3H6N6) in a He atmosphere. Surprisingly, the small compounds, such as Sc3N@C68, Sc3N@C70, Sc3N@C72, and Sc3N@C74, exhibit higher relative abundance than Sc3N@C80 and similar sized cages. That Sc3N@C80 nanocluster, however, displays an enhanced abundance compared to other medium-sized cages and is a “magic numbered” species. Under the present clusterfullerene-generating conditions, empty cage fullerenes are suppressed by laser synthesis, similar to observations for arc discharge synthesis from doped graphite containing these particular starting materials. Notably, all Sc3N@C2n (C2n = 68, 70, 78, 80, 82) synthesized and isolated by means of the arc discharge methods correspond to the observed cage sizes2, 29.
Fig. 1.
Clusterfullerenes formed by laser vaporization of group 3 metal-doped and nitrogen-doped graphite. a Synthesis schematic for clusterfullerenes formed from a mixture of graphite, metal oxide, and melamine (nitrogen source) in this work. FT-ICR mass spectra of cluster cations generated by laser vaporization of b Sc-doped and N-doped graphite and c Y-doped and N-doped graphite. M3N@C2n formation distributions are graphically shown below each spectrum
Although the smallest species, Sc3N@C62-C66, are formed in abundance under the present conditions, they may “react away” in the solid state or upon exposure to air or solvent. Therefore, some of these pristine isomers may not be readily detectable in arc discharge extracts or soot, as for other smaller non-IPR fullerenes30. Another possibility is that more carbon vapor may be available for insertion reactions in arc discharge than for the present laser vaporization conditions. However, it is clear that the Sc3N@C80 clusterfullerene is an abundantly produced medium-sized Sc3N@C2n formed by laser vaporization of doped graphite. At lower laser fluence (~5 mJ), we find that the smallest clusterfullerene cages, Sc3N@C2n (C2n = C62 to ~C74), are formed but medium-sized species, such as Sc3N@C80, are not observed. These results show that the smallest Sc3N-based clusterfullerenes appear to form before the larger compounds from doped graphite under our conditions.
Structural analysis of Sc3N@C2n produced from the bulk graphite-based starting material is obtained by collision-induced dissociation (CID) experiments, performed by means of sustained off-resonance irradiation (SORI)31. Supplementary Fig. 1 identifies the fragmentation pattern of gas-phase isolated Sc3N@C70 formed by laser vaporization of the graphite/Sc2O3/melamine mixture. The singular dissociation pathway observed for Sc3N@C70 in an ultrahigh vacuum is a C2-elimination event. The internally bound cluster, Sc3N, remains trapped within the nanoscale void of the carbon cage when highly thermally excited by collisions, whereas any exohedrally bound metals or heteroatoms would readily dissociate. Therefore, dissociation investigations provide compelling evidence that Sc3N@C70 exhibits a NCF structure. Larger Sc3N@C2n formation products display that same characteristic clusterfullerene dissociation pattern.
To discern the influence of the M3N cluster size effect with respect to the step of initial cage nucleation by means of bottom-up formation from starting material plasma, Y-doped melamine-containing graphite is examined under identical conditions. The larger ionic radius of Y (0.90 Å), and thus larger M3N cluster, compared to Sc (0.75 Å), provides a mechanistic avenue to experimentally probe that process. A striking change in formation distribution is observed for Y3N@C2n compared to Sc3N@C2n (Fig. 1). The small M3N-based clusterfullerenes observed for Sc3N@C2n are entirely absent, and, instead, nanocarbons are shifted to cage sizes of Y3N@C74 to Y3N@C88 32. However, the Y3N@C80 molecular ion, like Sc3N@C80, exhibits higher relative abundance indicating formation of a stable C80 isomer. The Y3N@C2n compounds, for example, Y3N@C80, are confirmed to be endohedral NCFs by SORI-CID investigations, as expected. Other families of metallofullerenes are detected from both Sc-doped and Y-doped carbon plasma systems and correspond to M2C2n, M3C2n, M4C2n, as well as odd carbon-numbered clusters such as M3Cn that must contain at least one C within a cage33. Thus, in addition to the successful formation of NCFs, numerous forms of clusterfullerenes are also produced from doped graphite by laser vaporization. Importantly, the observed cage size shift for Sc3N compared to Y3N clarifies that the M3N cluster nucleates formation of the smallest cages in the first bottom-up step.
Sc3N@C2n are formed in higher relative abundance than Y3N@C2n generated by laser vaporization of the starting materials, and therefore Sc3N is more efficiently entrapped within cages under our conditions. Thus, Sc appears to possess a special ability to bond with C and/or N in the initial nucleation step. Moreover, molecular ions that contain up to four Sc atoms are readily observed from Sc/N/C condensing plasma, whereas at most three Y atoms can be encapsulated from the Y/N/C plasma under the same vaporization parameters. These results provide mechanistic insight into how Sc may “pull in” other elements into cages through initial formation of the smallest NCFs34, which we propose grow into larger species through bottom-up reactions as we clearly demonstrate below.
Transformation of Sc3N@D3-C68 into Sc3N@C80
Chemical processes involved in the growth of the initially formed, small Sc3N-based clusterfullerenes from bulk starting materials are elucidated by analysis of isomerically pure NCF cages in carbon vapor generated from graphite, conducted under the same high energy formation conditions (10 mJ per pulse). These studies also provide insight into structural effects with respect to nanocarbon reactions that operate during self-assembly. Figure 2 shows that Sc3N@D 3-C68 unambiguously exhibits bottom-up growth by carbon insertion reactions that results in formation of larger Sc3N@C2n (C2n = 70–94) compounds. Under the present conditions, the most abundant growth product is Sc3N@C80, which exhibits an enhanced abundance compared to other medium-sized cages, similar to Sc3N@C80 generated by laser vaporization of bulk doped graphite (Fig. 1). Thus, in both cases, the results indicate that a stable, high-symmetry C80 cage isomer is formed. Dissociation investigations confirm that Sc3N is encapsulated in clusterfullerene growth product cages (Supplementary Figs. 2, 3).
Fig. 2.
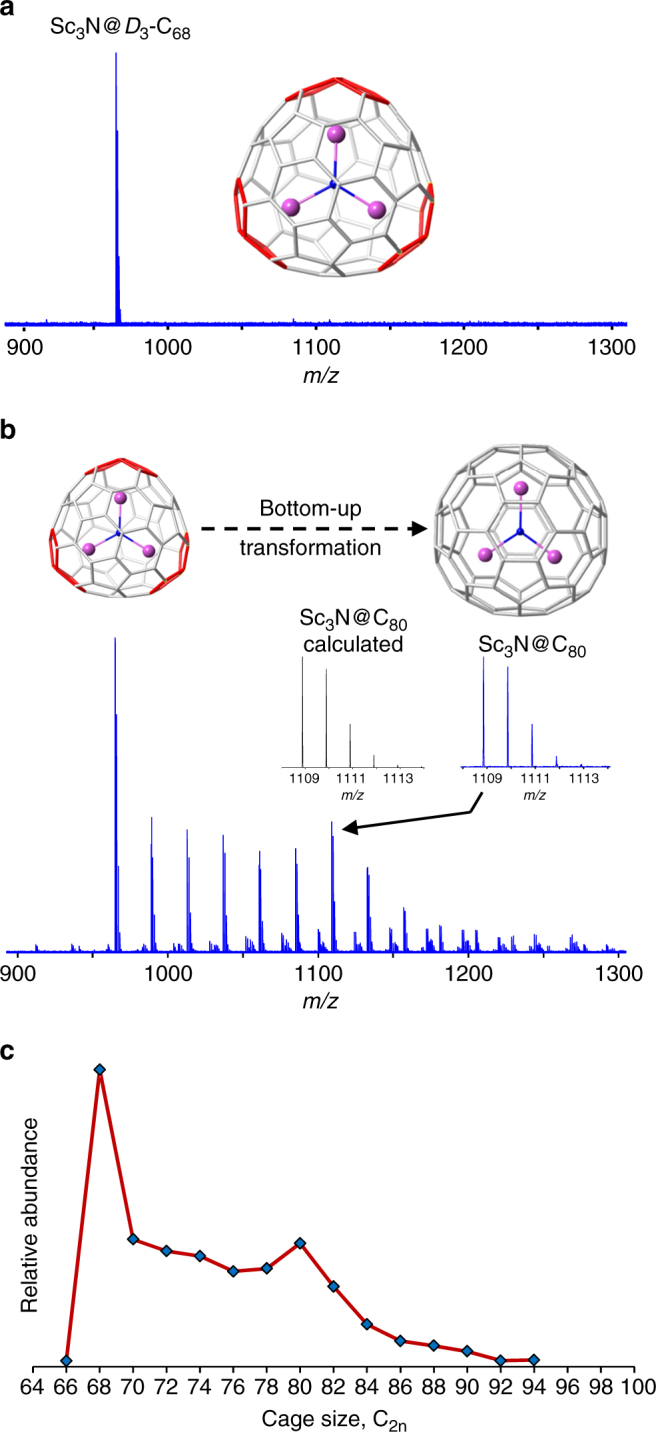
Bottom-up growth of a small, fused pentagon-containing clusterfullerene. a Low energy (~2 mJ) laser desorption spectrum (positive ions) of isomerically pure, Sc3N@D 3-C68, without exposure to carbon vapor from graphite. b Molecular reactivity and behavior of Sc3N@C68 in carbon vapor from graphite in a He atmosphere (~10 mJ per pulse). c Growth distribution for Sc3N@C68 + nC2
To distinguish formation mechanisms for the small Sc3N@C2n (C2n = C62–C66) products synthesized by laser vaporization of Sc-doped and N-doped graphite, which have not been detected in extracts or soot from arc discharge, Sc3N@D 3-C68 is studied in the absence of graphite vapor under the high energy conditions of synthesis. Supplementary Fig. 4 shows products generated from Sc3N@D 3-C68 by direct laser ablation (10 mJ per pulse) without exposure to carbon vapor. Surprisingly, Sc3N@C70, a bottom-up growth structure, is the most abundant molecular reaction product, suggesting that carbon insertion reactions can be favored in low carbon density, high-energy conditions. Sc2N@C68, a Sc-loss product, and Sc3N@C66, formed by C2-loss, are observed only in very low abundance. Consequently, these results provide additional evidence that the smallest Sc3N@C2n (C2n = C62–C66) are not formed from larger Sc3N@C2n by top-down processes during self-assembly from graphite starting material and are consistent with a bottom-up formation mechanism.
Reaction products that correspond to non-NCF compounds, namely, Sc3NCn, Sc2NCn, and ScNCn (Cn = odd number of C atoms) also result after growth of Sc3N@D 3-C68 in carbon vapor (Supplementary Fig. 5). We find that all odd-numbered carbon chemical compositions dissociate by C2-elimination with retention of an odd number of carbon and all Sc and N atoms. Consequently, the presence of a carbon adatom is excluded because it readily dissociates at low thermal energy. Therefore, all of these molecular products contain clusters of Sc, N, and C within cages. As a result of the present high energy formation conditions that render compounds with no C adatoms, the Sc3NCn species plausibly exhibit structures, Sc3NC@C2n, in which the five-atom cluster, Sc3NC, is entrapped within even-numbered carbon cages. These species result from intramolecular reactions that take place during the growth process. Notably, the smallest member of Sc3NCn observed from Sc3N@D 3-C68 growth is Sc3NC81 (Supplementary Fig. 5), which may be a contributing formation route to Sc3NC81 that has been isolated by means of arc discharge and exhibits a carbonitride clusterfullerene structure, Sc3NC@C80 35.
Computational analysis for reaction paths from C68 to C80
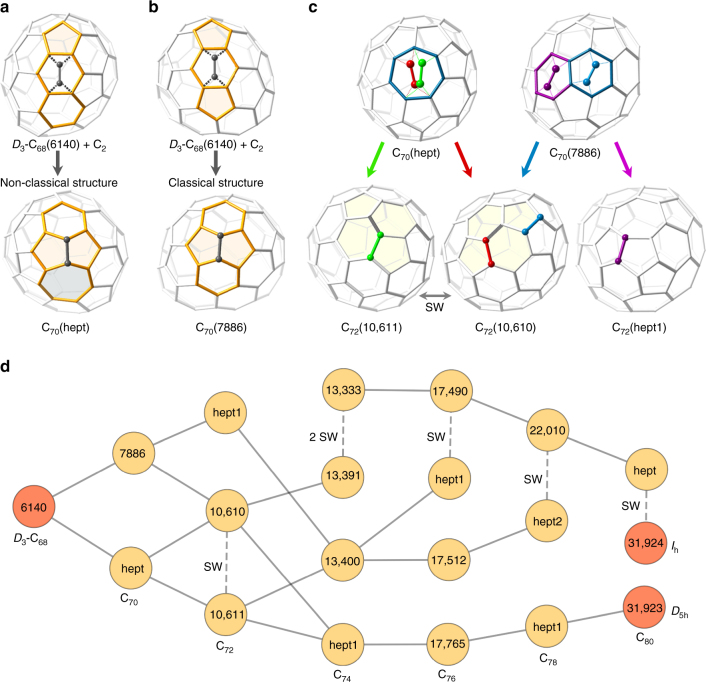
Sc3N@D 3-C68 is clearly demonstrated to transform into Sc3N@C70 after exposure to carbon vapor generated from graphite, which involves the overall incorporation of C2 into the caged network. That nanocluster is also among the most abundant NCFs formed by laser vaporization of Sc-doped and N-doped graphite in this work. Interestingly, the only Sc3N@C70 species that has been isolated is C70(7854)36. Note that we will now use the spiral algorithm numerical identifier in parenthesis to distinguish isomers for a particular cage size37. To discern a reaction path for the Sc3N@D 3-C68 to Sc3N@C70 transformation, all possible topological C2 insertions were analyzed for the D 3-C68(6140) cage in the hexaanionic form, C2n 6−, to account for charge transfer from the encapsulated Sc3N cluster to the cage. Six isomers of C70 can be generated by direct C2 insertion (Supplementary Fig. 6) without the involvement of Stone-Wales (SW) bond rearrangements. The two lowest energy product structures, shown in Fig. 3a, b, are found to be associated with very exothermic energies (Supplementary Table 1). C70(7886) exhibits a classical structure comprised of pentagons and hexagons, whereas C70(hept) possesses a non-classical structure that contains a heptagon motif.
Fig. 3.
Reaction paths to high-symmetry C80 isomers (I h, D 5h) from D 3-C68 through non-classical and classical cages. D 3-C68 can grow by C2 insertion into the a non-classical structure that contains a heptagon motif, C70(hept), or b a classical structure comprised of only pentagons and hexagons, C70(7886). c For the next bottom-up cage transformation, C70 to C72, the most plausible isomers are found to be the classical cages, C72(10611), C72(10610), and heptagon-containing C72(hept1). d From those C72 isomers, reaction paths to high-symmetry C80 cages, I h-C80 and D 5h-C80, involve C2 insertion reactions and two to three SW rearrangements through classical and non-classical cage intermediates
Analysis of all possible C2 insertions into these predicted C70 isomers (Supplementary Fig. 7) is performed for the next bottom-up cage transformation and plausible C72 intermediates are found to be C72(10611), C72(10610), and C72(hept1), as shown in Fig. 3c. Notably, C72(10611) acts as a “gateway” cage and is intimately related by a single SW rearrangement (Supplementary Fig. 8) to C72(10610) with a barrier (~150 kcal mol−1) that can be surpassed at the temperature of fullerene formation (>1000 K). Although the heptagon structure is 24.2 kcal mol−1 higher in energy than C72(106011), we find that the energy profiles for these cage growth transformations are similar (Supplementary Fig. 9). Therefore, formation of classical and heptagon-containing cages should take place during bottom-up clusterfullerene growth, which is in agreement with the recent characterization of trimetallic nitride, LaSc2N@C80(hept), as well as for larger heptagon-containing cage sizes15, 38, 39. Structures that possess two heptagons are not considered because such cages are very strained and are at least 54 kcal mol−1 higher in energy than C72(10611). Reaction energies for all of the lowest energy isomers were computed in the clusterfullerene form for comparison to the hexaanionic investigations. Supplementary Table 2 shows that reaction energies are somewhat lower, but remain very exothermic. Thus, formation of the proposed C70 and C72 growth structures is favorable based on thermodynamic considerations. A detailed description of the growth mechanism is given in Supplementary Fig. 9.
We have investigated global pathways to high-symmetry C80 clusterfullerenes from Sc3N@D 3-C68 based on this strategy for step-by-step cage formations of low energy isomers through C2 insertions and bond rearrangements. Despite the complexity of the processes involved (Supplementary Fig. 10), it is extraordinary that several relatively simple pathways exist and are shown in Fig. 3d. Initiating from the proposed C68 to C72 structure progression, we find reaction paths from D 3-C68 to icosahedral C80 that involve the known NCF cages, C78(22010), C80(hept), and C80(I h) in final reaction sequence, where C80(hept) is the structure of recently isolated NCF, LaSc2@C80(hept)15, 40–42. Strikingly, Fig. 3d also shows that a bottom-up sequence of six direct C2 insertions without any SW rearrangement results in the known high-symmetry D 5h-C80 cage, M3N@D 5h-C80(31923). Thus, we find that all plausible bottom-up growth routes to high-symmetry C80 involve a total of six C2 insertion reactions and two to three C2 rearrangements. Importantly, reaction energies, and free energies computed at the high temperature of synthesis (2000 K), for all routes are confirmed to be very exothermic (Supplementary Tables 2–4). It is possible that some of these predicted non-classical and classical intermediates may be isolated and characterized in the near future (Fig. 3, Supplementary Fig. 10, and Supplementary Table 5).
Growth of M3N@C80 into larger, lower symmetry cages
As a crucial test for our proposed bottom-up formation mechanism from graphite, the M3N@I h-C80 compound is specifically investigated under the harsh conditions typical of plasma synthesis, thereby providing important nanocarbon mechanistic information because of its icosahedral symmetry and medium cage size. Figure 4a–c shows the products formed after exposure of isomerically pure Sc3N@I h-C80 to carbon plasma in He. An identical laser fluence (10 mJ per pulse) is used for all molecular reactivity studies in this work to facilitate comparison to gas-phase synthesis of Sc3N@C2n from raw starting materials without pre-existing NCFs. Numerous larger metallic nitride species are formed by repeated carbon insertion reactions into the Sc3N@I h-C80 precursor. The dominant nanocarbon reaction after exposure of Sc3N@I h-C80 to graphite vapor is a single C2 insertion to yield Sc3N@C82. By contrast, species smaller than Sc3N@C80 are absent, including Sc3N@C68, revealing that carbon loss or top-down formation to smaller cages is not a significant process under the high energy, high carbon density conditions of synthesis. The structures of the molecular product ions Sc3N@C2n (C2n ≥ 82) formed through bottom-up growth of Sc3N@I h-C80 are confirmed (Supplementary Fig. 11) to be trimetallic NCFs. In addition, we find that carbon loss reactions do not readily take place when Sc3N@I h-C80 is subjected to high energy laser ablation (10 mJ per pulse) without carbon vapor, i.e., high energy, low carbon density conditions (Supplementary Fig. 12), a molecular behavior consistent with the much smaller Sc3N@D 3-C68. The D 5h-C80 isomer encapsulated by Sc3N was also probed in graphite vapor and exhibits bottom-up growth behavior, and it appears to be slightly more reactive than I h-C80 (Supplementary Fig. 13).
Fig. 4.
Influence of the encapsulated cluster on growth of icosahedral C80. Isomerically pure a Sc3N@I h-C80 after laser desorption (~2 mJ) without carbon vapor and b after reaction with graphite vapor in He (10 mJ) and c Sc3N@C2n + nC2 formation distribution. Comparison of isomerically pure d Y3N@I h-C80 after laser desorption (~2 mJ) and e reaction with graphite vapor under identical conditions (10 mJ) and f Y3N@C2n + nC2 formation distribution. C2-elimination events are not readily observed for either M3N@I h-C80
To probe cluster-cage size effects with respect to carbon insertion reactions for the I h-C80 cage, molecular reactivity studies are performed whereby Y3N is substituted for Sc3N. Figure 4d–f shows products that result after exposure of isomerically pure Y3N@I h-C80 to graphite vapor, conducted under identical conditions to those for the Sc3N@I h-C80 (and Sc3N@D 3-C68) growth experiments. Y3N@C82, formed by C2 incorporation, is formed in high relative abundance; however, Y3N@C84 and Y3N@C2n compounds as large as Y3N@C100 are present in substantial abundance. In addition, Y3N@I h-C80, like Sc3N@I h-C80, does not readily exhibit carbon loss events or top-down behavior (Supplementary Fig. 14) when subjected to high energy laser ablation in absence of carbon vapor (i.e., low carbon density, high energy conditions). Extensive SORI-CID experiments strongly support that the major growth products are homogeneous Y-based NCFs (Supplementary Fig. 15). The more extensive bottom-up growth of Y3N@I h-C80 is in agreement with the formation distribution of Y3N@C2n generated from Y-doped and N-doped graphite (Fig. 1). Thus, the bottom-up mechanism is attributed to also operate in the Y-containing graphite starting material plasma.
Further evidence to corroborate metallic NCF structures for Y3N@C2n growth products, as well as Sc3N@C2n, synthesized in this work is obtained by dissociation analysis of the endohedral heterofullerene, Y2@C79N, which exhibits an 80 atom cage that substitutes N for a single C atom in the caged network43. We find that the dissociation route for Y2@C79N is CN-elimination (Supplementary Fig. 16), in contrast to C2-loss with retention of N for the Y3N@C80 endohedral nanocluster. These results are consistent with empty cage N-containing heterofullerenes44. Thus, the present gas-phase dissociation studies are shown to be a structural diagnostic for identification of clusterfullerenes and endohedral heterofullerenes.
The mechanistic uniqueness of Sc3N@I h-C80 is clearly established in this work by its lack of growth into larger species, whereas Y3N@I h-C80 clearly diverges from that trend and can grow into cages that exceed 100 carbon atoms in size. These results also support the assignment of a high-symmetry cage, such as I h-C80, as a major contributing isomer for the magic numbered Sc3N@C80 nanocluster formed from bulk starting materials by laser synthesis, although other isomers should be produced to a lesser extent. Pyramidalization of carbon atoms takes place when a metal is coordinated to the [5,6] carbon atoms for Sc3N@C80 compared to the center of a hexagon for Y3N@C80 45. Consequently, the pyrene-type carbon atoms in Y3N@C80 are more strained and may be more reactive to carbon insertion reactions. It is noteworthy that the M3N-encapsulated product cages shown in Fig. 4 must be lower symmetry than the I h-C80 precursor. Interestingly, the small non-IPR Sc3N@D 3-C68 compound that contains three sets of fused pentagons appears to be somewhat less reactive in the bottom-up growth scheme than Y3N@I h-C80. That result distinguishes how the transfer of six electrons and an optimally sized M3N cluster significantly renders non-IPR cages less reactive through bottom-up mechanisms. Those observations further account for the different formation trends for Sc3N@C2n and Y3N@C2n from the original doped graphite.
Discussion
We propose that high-symmetry clusterfullerenes primarily self-assemble through a bottom-up mechanism by simple vaporization of graphite. Online chemical sampling of laser vaporized doped graphite reveals an initial nucleation step, whereby M3N nucleates formation of the smallest possible cage(s). For example, Sc3N bypasses cages smaller than C62, whereas the larger Y3N cluster must initially nucleate cages of C74 or larger under our conditions. Those results clarify that formation initiates through bottom-up processes that exhibit a cluster size effect on cage selection. Avoidance of I h-C60 in the bottom-up mechanism and thus the many other small “bottleneck” cages, C28, C36, C44, C50, in reaction paths that severely limit growth to medium-sized conventional metallofullerenes, M@C2n 23, 46, enable clusterfullerene cages in the solvent-extractable ~C70–C100 region to form in inherently higher abundance. Further, that mechanistic property allows breakthrough access to the next possible icosahedral cage, I h-C80, which may then act as a mechanistic bottleneck in formation for group 3 NCFs, and, in particular, should permit Sc3N@I h-C80 to accumulate in bottom-up reaction paths and thus explains its high-yield formation.
Our bottom-up model for NCF formation is further experimentally tested by extensive investigations on specified isomers of the group 3-based clusterfullerenes, Sc3N@D 3-C68, Sc3N@I h-C80, Sc3N@D 5h-C80, and Y3N@I h-C80 by exposure to graphite vapor under characteristic physicochemical synthetic conditions. Fused pentagon-containing Sc3N@C68 unambiguously grows into Sc3N@C80, which exhibits an enhanced abundance in agreement with the proposal that medium-sized Sc3N@C2n from bulk doped graphite primarily form through a bottom-up mechanism. The archetypal clusterfullerene, Sc3N@I h-C80, is observed to be rather inert to further bottom-up growth, which is consistent with the assignment of I h-C80 for Sc3N@C80 from doped graphite and from the explicit growth of Sc3N@D 3-C68 in this work. Theoretical investigations show that cage transformations from D 3-C68 cage into I h-C80 can occur by a total of six C2 insertions and only two to three SW rearrangements. Heptagon-containing and classical cages are predicted to be involved in these transformations. The D 5h-C80 isomer can self-assemble without any SW rearrangements through six direct C2 insertion events.
Substitution of Y3N in the I h-C80 cage dramatically alters the molecular behavior of I h-C80 in graphite vapor and Y3N@C80 readily grows into clusterfullerenes that exhibit 100 carbon atom cages. That cluster-cage size effect further explains the shift to larger cages in formation distributions for Sc3N@C2n to Y3N@C2n from doped graphite. Unexpectedly, we find that NCFs can transform into five-atom cluster entrapped cages that presumably result from intramolecular cage reactions that take place during bottom-up growth of Sc3N@C68. For example, endohedral species with a chemical composition of Sc3NC81 are formed that may be the origin of five-atom cluster encapsulated, Sc3NC@C80, and thus offers another mechanistic route to clusterfullerene formation through bottom-up growth paths. We do not observe formation of M3NC81 by growth of the larger Sc3N@I h-C80, Sc3N@D 5h-C80, or Y3N@I h-C80 clusterfullerenes NCFs under the present conditions, suggesting that cluster interaction with the cage during bottom-up growth could be crucial to its formation.
In conclusion, through in situ investigations of clusterfullerenes synthesized by laser vaporization of doped graphite and study of discrete NCF compounds, combined with extensive theoretical investigations, we disclose that bottom-up self-assembly reactions are responsible for synthesis of high-symmetry carbon cages that encapsulate metallic nitride clusters. We propose that these intrinsic chemical processes are a fundamental property of carbon under harsh conditions typical of synthesis and will help tackle the challenge of carbon nanostructure formation for other hybrid carbon allotropes and should be useful for synthesis of new carbon-based cluster compounds. We note that it is possible that a “local” C2-loss event may occur in a “global” bottom-up path or in the solid state after formation, and therefore should also be considered to comprehensively describe fullerene formation. Future in situ studies of doped graphite, combined with the analysis of distinct clusterfullerenes, are now possible for compounds that encapsulate other endohedral clusters (e.g., metal carbides, sulfides, oxides, etc.)47–53 and possess various structural motifs, which should facilitate the exploration of new forms of encapsulated nanocarbon materials and their fundamental self-assembly processes.
Methods
Clusterfullerenes from doped graphite
Doped graphite starting materials are produced by physical mixing of graphite (99.9995%, 2−15 μm), scandium oxide or yttrium oxide (99.9%), and melamine (99%)23. The doped graphite material is then molded into a 12.7-mm rod by compression of the mixture. Doped graphite rods are comprised of 10 atom % metal, with a 1:2 ratio for metal:C3H6N6.
Reactivity of cluster-encapsulated cages in graphite vapor
Macroscopic samples of metallic NCFs are synthesized by arc discharge. Isomerically pure samples of Sc3N@I h-C80 and Sc3N@D 3-C68 were purified by use of non-chromatographic methods54. Y3N@I h-C80 was purified by multi-stage HPLC. Isomerically pure NCF was then uniformly applied to the surface of a pristine 12.7-mm graphite rod (99.9995%, 2−15 μm) for nanocarbon reaction studies by use of a Nd:YAG (532 nm, 10 mJ) pulsed laser cluster source23, 25. NCFs were individually applied to a quartz rod for high energy, direct laser ablation without exposure to carbon plasma.
Cluster source and 9.4 T FT-ICR mass spectrometry
Self-assembly reaction experiments are analyzed with a custom-built FT-ICR mass spectrometer based on a 9.4 T superconducting magnet and performed with positive ions produced by a pulsed laser cluster source23, 24. Evaporation of a doped graphite stationary rod (12.7-mm diameter) is achieved by a single laser shot fired from a Nd:YAG (532 nm, 3–5 ns pulse width, 1.5-mm beam diameter) in conjunction with the opening of a pulsed valve (800 ms duration) to admit He flow over the sample. Carbon vapor produced then enters a channel 4 mm in diameter and ~8.5 mm in length. The laser is fired ~2 ms after opening of the pulsed valve for evaporation of doped graphite samples and ~4 ms for Sc3N@C68, Sc3N@I h-C80, Sc3N@D 5h-C80, and Y3N@I h-C80-coated graphite samples. Ions accumulated by ten individual laser and helium pulse events are transported to an open cylindrical ion trap (70-mm diameter, 212-mm long, aspect ratio ~2). The ions are accelerated to a detectable cyclotron radius by a broadband frequency sweep excitation (260 Vp-p, 150 Hz µs−1, 3.6 down to 0.071 MHz) and subsequently detected as the differential current induced between two opposed electrodes of the ICR cell. Each of the acquisitions is Hanning-apodized and zero-filled once before fast Fourier transform and magnitude calculation23. Up to ten time-domain acquisitions are averaged. The experimental event sequence is controlled by a modular ICR data acquisition system. Ions are further probed by CID.
Computational details
Amsterdam Density Functional code (ADF2012) was used for the electronic structure calculations55. The electronic density was provided by the local density approximation by use of Becke’s gradient corrected exchange functional, and Vosko, Wilk, Nusair parametrization for correlation, corrected with Perdew’s functional (BP86). Electrons for all the atoms were described with Slater-type basis functions of triple-ζ + polarization quality. We have included scalar relativistic corrections by means of the zeroth-order regular approximation formalism. All Sc3N@C2n calculations have also been performed including dispersion corrections. We used the CaGe program to generate fullerenes and Schlegel diagrams.
Data availability
Data that support the findings of this study are available within the paper and its supplementary information files, and available from the corresponding author upon request. A data set collection of computational results is available from the online ioChem-BD repository and can be accessed via https://doi.org/10.19061/iochem-bd-2–16, where all intermediates described in Fig. 3 and Supplementary Fig. 10 can be found.
Electronic supplementary material
Acknowledgements
Work performed at the National High Magnetic Field Laboratory is supported by the NSF Cooperative Agreement through DMR-11-57490 and the State of Florida. P.W.D. thanks the FSU Research Foundation for support. This work was also supported by the Spanish Ministerio de Ciencia e Innovación (CTQ2014-52774-P) and the Generalitat de Catalunya (2014SGR-199 and XRQTC). L.A. thanks the GC for a predoctoral fellowship (FI-DGR 2014). L.E. thanks the NSF for generous support under PREM program (DMR 1205302) and CHE-140885, and the Robert A. Welch Foundation (Grant AJ-0033). We thank Prof. Gunnar Brinkmann for helpful assistance in the use of CaGe software. We thank John Quinn and Greg Blakney for instrument assistance, and Chad Weisbrod for discussion. Dedicated to the memory of Harry Kroto.
Author contributions
P.W.D., A.R.-F., J.M.P., and L.E. designed the research and wrote the paper; M.M.-G. performed the experiments under the supervision of P.W.D.; L.A., A.R.-F., and J.M.P. designed and performed the theoretical calculations; M.R.C. and E.C. synthesized and purified samples used in the experiments; M.M.-G., L.A., A.G.M., A.R.-F., L.E., J.M.P., and P.W.D. analyzed data. All authors discussed the results and commented on the manuscript.
Competing interests
The authors declare no competing financial interests.
Footnotes
Marc Mulet-Gas and Laura Abella contributed equally to this work.
Electronic supplementary material
Supplementary Information accompanies this paper at doi:10.1038/s41467-017-01295-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Luis Echegoyen, Email: echegoyen@utep.edu.
Josep M. Poblet, Email: josepmaria.poblet@urv.cat
Paul W. Dunk, Email: dunk@magnet.fsu.edu
References
- 1.Stevenson S, et al. Small-bandgap endohedral metallofullerenes in high yield and purity. Nature. 1999;401:55–57. doi: 10.1038/43415. [DOI] [Google Scholar]
- 2.Popov AA, Yang SF, Dunsch L. Endohedral fullerenes. Chem. Rev. 2013;113:5989–6113. doi: 10.1021/cr300297r. [DOI] [PubMed] [Google Scholar]
- 3.Wang TS, Wang CR. Endohedral metallofullerenes based on spherical Ih-C80 cage: molecular structures and paramagnetic properties. Acc. Chem. Res. 2014;47:450–458. doi: 10.1021/ar400156z. [DOI] [PubMed] [Google Scholar]
- 4.Ross RB, et al. Endohedral fullerenes for organic photovoltaic devices. Nat. Mater. 2009;8:208–212. doi: 10.1038/nmat2379. [DOI] [PubMed] [Google Scholar]
- 5.Zhang JY, et al. Gd3N@C84(OH)x: a new egg-shaped metallofullerene magnetic resonance imaging contrast agent. J. Am. Chem. Soc. 2014;136:2630–2636. doi: 10.1021/ja412254k. [DOI] [PubMed] [Google Scholar]
- 6.Rincon-Garcia L, et al. Molecular design and control of fullerene-based bi-thermoelectric materials. Nat. Mater. 2016;15:289–293. doi: 10.1038/nmat4487. [DOI] [PubMed] [Google Scholar]
- 7.Fatouros PP, et al. In vitro and in vivo imaging studies of a new endohedral metallofullerene nanoparticle. Radiology. 2006;240:756–764. doi: 10.1148/radiol.2403051341. [DOI] [PubMed] [Google Scholar]
- 8.Zhang JY, Stevenson S, Dorn HC. Trimetallic nitride template endohedral metallofullerenes: discovery, structural characterization, reactivity, and applications. Acc. Chem. Res. 2013;46:1548–1557. doi: 10.1021/ar300301v. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Fortea A, Balch AL, Poblet JM. Endohedral metallofullerenes: a unique host-guest association. Chem. Soc. Rev. 2011;40:3551–3563. doi: 10.1039/c0cs00225a. [DOI] [PubMed] [Google Scholar]
- 10.Popov AA, Dunsch L. Structure, stability, and cluster-cage interactions in nitride clusterfullerenes M3N@C2n (M=Sc, Y; 2n=68–98): a density functional theory study. J. Am. Chem. Soc. 2007;129:11835–11849. doi: 10.1021/ja073809l. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Fortea A, Alegret N, Balch AL, Poblet JM. The maximum pentagon separation rule provides a guideline for the structures of endohedral metallofullerenes. Nat. Chem. 2010;2:955–961. doi: 10.1038/nchem.837. [DOI] [PubMed] [Google Scholar]
- 12.Stevenson S, et al. Materials science—a stable non-classical metallofullerene family. Nature. 2000;408:427–428. doi: 10.1038/35044199. [DOI] [PubMed] [Google Scholar]
- 13.Wang CR, et al. Materials science—C66 fullerene encaging a scandium dimer. Nature. 2000;408:426–427. doi: 10.1038/35044195. [DOI] [PubMed] [Google Scholar]
- 14.Yamada M, et al. Sc2@C66 revisited: an endohedral fullerene with scandium ions nestled within two unsaturated linear triquinanes. J. Am. Chem. Soc. 2014;136:7611–7614. doi: 10.1021/ja5035649. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, et al. Synthesis and structure of LaSc2N@Cs(hept)-C80 with one heptagon and thirteen pentagons. Angew. Chem. Int. Ed. 2015;54:495–499. doi: 10.1002/anie.201409094. [DOI] [PubMed] [Google Scholar]
- 16.Zhang JY, et al. A missing link in the transformation from asymmetric to symmetric metallofullerene cages implies a top-down fullerene formation mechanism. Nat. Chem. 2013;5:880–885. doi: 10.1038/nchem.1748. [DOI] [PubMed] [Google Scholar]
- 17.Cai WT, Li FF, Bao LPA, Xie YP, Lu X. Isolation and crystallographic characterization of La2C2@Cs(574)-C102 and La2C2@C2(816)-C104: evidence for the top-down formation mechanism of fullerenes. J. Am. Chem. Soc. 2016;138:6670–6675. doi: 10.1021/jacs.6b03934. [DOI] [PubMed] [Google Scholar]
- 18.Irle S, Zheng GS, Wang Z, Morokuma K. The C60 formation puzzle “solved”: QM/MD simulations reveal the shrinking hot giant road of the dynamic fullerene self-assembly mechanism. J. Phys. Chem. B. 2006;110:14531–14545. doi: 10.1021/jp061173z. [DOI] [PubMed] [Google Scholar]
- 19.Chuvilin A, Kaiser U, Bichoutskaia E, Besley NA, Khlobystov AN. Direct transformation of graphene to fullerene. Nat. Chem. 2010;2:450–453. doi: 10.1038/nchem.644. [DOI] [PubMed] [Google Scholar]
- 20.Greisch JF, et al. From planar to cage in 15 easy steps: resolving the C60H21F9- - > C60- transformation by ion mobility mass spectrometry. J. Am. Chem. Soc. 2016;138:11254–11263. doi: 10.1021/jacs.6b06205. [DOI] [PubMed] [Google Scholar]
- 21.Kroto HW, Heath JR, Obrien SC, Curl RF, Smalley RE. C60—buckminsterfullerene. Nature. 1985;318:162–163. doi: 10.1038/318162a0. [DOI] [Google Scholar]
- 22.Yang S, Zhang L, Zhang W, Dunsch L. A facile route to metal nitride clusterfullerenes by using guanidinium salts: a selective organic solid as the nitrogen source. Chem. Eur. J. 2010;16:12398–12405. doi: 10.1002/chem.201001252. [DOI] [PubMed] [Google Scholar]
- 23.Dunk PW, et al. Bottom-up formation of endohedral mono-metallofullerenes is directed by charge transfer. Nat. Commun. 2014;5:5844. doi: 10.1038/ncomms6844. [DOI] [PubMed] [Google Scholar]
- 24.Duncan MA. Invited review article: laser vaporization cluster sources. Rev. Sci. Instrum. 2012;83:041101. doi: 10.1063/1.3697599. [DOI] [PubMed] [Google Scholar]
- 25.Dunk PW, et al. Closed network growth of fullerenes. Nat. Commun. 2012;3:855. doi: 10.1038/ncomms1853. [DOI] [PubMed] [Google Scholar]
- 26.Dunsch L, et al. Metal sulfide in a C82 fullerene cage: a new form of endohedral clusterfullerenes. J. Am. Chem. Soc. 2010;132:5413–5421. doi: 10.1021/ja909580j. [DOI] [PubMed] [Google Scholar]
- 27.Svitova AL, et al. Endohedral fullerene with u3-carbido ligand and titanium-carbon double bond stabilized inside a carbon cage. Nat. Commun. 2014;5:3568. doi: 10.1038/ncomms4568. [DOI] [PubMed] [Google Scholar]
- 28.Svitova AL, Popov AA, Dunsch L. Gd-Sc-based mixed-metal nitride cluster fullerenes: mutual influence of the cage and cluster size and the role of scandium in the electronic structure. Inorg. Chem. 2013;52:3368–3380. doi: 10.1021/ic400049k. [DOI] [PubMed] [Google Scholar]
- 29.Wei T, et al. Capturing the long-sought small-bandgap endohedral fullerene Sc3N@C82 with low kinetic stability. J. Am. Chem. Soc. 2015;137:3119–3123. doi: 10.1021/jacs.5b00199. [DOI] [PubMed] [Google Scholar]
- 30.Tan YZ, Xie SY, Huang RB, Zheng LS. The stabilization of fused-pentagon fullerene molecules. Nat. Chem. 2009;1:450–460. doi: 10.1038/nchem.329. [DOI] [PubMed] [Google Scholar]
- 31.Marshall AG, Hendrickson CL, Jackson GS. Fourier transform ion cyclotron resonance mass spectrometry: a primer. Mass Spectrom. Rev. 1998;17:1–35. doi: 10.1002/(SICI)1098-2787(1998)17:1<1::AID-MAS1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 32.Melin F, et al. The large Nd3N@C2n (40 < n < 49) cluster fullerene family: preferential templating of a C88 cage by a trimetallic nitride cluster. Angew. Chem. Int. Ed. 2007;46:9032–9035. doi: 10.1002/anie.200703489. [DOI] [PubMed] [Google Scholar]
- 33.Sarina EA, et al. 2-aminoethanol extraction as a method for purifying Sc3N@C80 and for differentiating classes of endohedral fullerenes on the basis of reactivity. Chem. Eur. J. 2015;21:17035–17043. doi: 10.1002/chem.201502415. [DOI] [PubMed] [Google Scholar]
- 34.Wei T, et al. Entrapping a group-VB transition metal, vanadium, within an endohedral metallofullerene: VxSc3-xN@Ih-C80 (x = 1, 2) J. Am. Chem. Soc. 2016;138:207–214. doi: 10.1021/jacs.5b10115. [DOI] [PubMed] [Google Scholar]
- 35.Wang T-S, et al. Planar quinary cluster inside a fullerene cage: synthesis and structural characterizations of Sc3NC@C80-Ih. J. Am. Chem. Soc. 2010;132:16362–16364. doi: 10.1021/ja107843b. [DOI] [PubMed] [Google Scholar]
- 36.Yang SF, Popov AA, Dunsch L. Violating the isolated pentagon rule (IPR): the endohedral non-IPR cage of Sc3N@C70. Angew. Chem. Int. Ed. 2007;46:1256–1259. doi: 10.1002/anie.200603281. [DOI] [PubMed] [Google Scholar]
- 37.Fowler, P. W. & Manolopoulos, D. E. An Atlas of Fullerenes (Oxford, Clarendon Press, 1995).
- 38.Chen C-H, et al. Zigzag Sc2C2 carbide cluster inside a [88]fullerene cage with one heptagon, Sc2C2@Cs(hept)-C88: a kinetically trapped fullerene formed by C2 insertion? J. Am. Chem. Soc. 2016;138:13030–13037. doi: 10.1021/jacs.6b07912. [DOI] [PubMed] [Google Scholar]
- 39.Gan LH, Lei D, Fowler PW. Structural interconnections and the role of heptagonal rings in endohedral trimetallic nitride template fullerenes. J. Comput. Chem. 2016;37:1907–1913. doi: 10.1002/jcc.24407. [DOI] [PubMed] [Google Scholar]
- 40.Kato H, Taninaka A, Sugai T, Shinohara H. Structure of a missing-caged metallofullerene: La2@C72. J. Am. Chem. Soc. 2003;125:7782–7783. doi: 10.1021/ja0353255. [DOI] [PubMed] [Google Scholar]
- 41.Yamada M, et al. Regioselective cage opening of La2@D2(10611)-C72 with 5,6-diphenyl-3-(2-pyridyl)-1,2,4-triazine. Angew. Chem. Int. Ed. 2015;54:2232–2235. doi: 10.1002/anie.201410012. [DOI] [PubMed] [Google Scholar]
- 42.Beavers CM, Chaur MN, Olmstead MM, Echegoyen L, Balch AL. Large metal Ions in a relatively small fullerene cage: the structure of Gd3N@C2(22010)-C78 departs from the isolated pentagon rule. J. Am. Chem. Soc. 2009;131:11519–11524. doi: 10.1021/ja903741r. [DOI] [PubMed] [Google Scholar]
- 43.Zuo TM, et al. M2@C79N (M = Y, Tb): isolation and characterization of stable endohedral metallofullerenes exhibiting M-M bonding interactions inside aza[80]fullerene cages. J. Am. Chem. Soc. 2008;130:12992–12997. doi: 10.1021/ja802417d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clipston NL, et al. Laser-induced formation, fragmentation, coalescence, and delayed ionization of the C59N heterofullerene. J. Phys. Chem. A. 2000;104:9171–9179. doi: 10.1021/jp001837j. [DOI] [Google Scholar]
- 45.Yang SF, Popov AA, Dunsch L. Carbon pyramidalization in fullerene cages induced by the endohedral cluster: non-scandium mixed metal nitride clusterfullerenes. Angew. Chem. Int. Ed. 2008;47:8196–8200. doi: 10.1002/anie.200802009. [DOI] [PubMed] [Google Scholar]
- 46.Dunk PW, et al. The smallest stable fullerene, M@C28 (M = Ti, Zr, U): stabilization and growth from carbon vapor. J. Am. Chem. Soc. 2012;134:9380–9389. doi: 10.1021/ja302398h. [DOI] [PubMed] [Google Scholar]
- 47.Tang QQ, et al. Sc2O@C2v(5)-C80: dimetallic oxide cluster inside a C80 fullerene cage. Inorg. Chem. 2015;54:9845–9852. doi: 10.1021/acs.inorgchem.5b01613. [DOI] [PubMed] [Google Scholar]
- 48.Wang TS, et al. Russian-doll-type metal carbide endofullerene: synthesis, isolation, and characterization of Sc4C2@C80. J. Am. Chem. Soc. 2009;131:16646–16647. doi: 10.1021/ja9077842. [DOI] [PubMed] [Google Scholar]
- 49.Stevenson S, et al. A distorted tetrahedral metal oxide cluster inside an icosahedral carbon cage. synthesis, isolation, and structural characterization of Sc4(μ3-O)2@Ih-C80. J. Am. Chem. Soc. 2008;130:11844–11845. doi: 10.1021/ja803679u. [DOI] [PubMed] [Google Scholar]
- 50.Nishibori E, et al. High-resolution analysis of (Sc3C2)@C80 metallofullerene by third generation synchrotron radiation X-ray powder diffraction. J. Phys. Chem. B. 2006;110:19215–19219. doi: 10.1021/jp061740i. [DOI] [PubMed] [Google Scholar]
- 51.Junghans K, Rosenkranz M, Popov AA. Sc3CH@C80: selective 13C enrichment of the central carbon atom. Chem. Commun. 2016;52:6561–6564. doi: 10.1039/C5CC10025A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iiduka Y, et al. Structural determination of metallofuIlerene Sc3C82 revisited: a surprising finding. J. Am. Chem. Soc. 2005;127:12500–12501. doi: 10.1021/ja054209u. [DOI] [PubMed] [Google Scholar]
- 53.Kurihara H, et al. Chemical understanding of carbide cluster metallofullerenes: a case study on Sc2C2@C2v(5)-C80 with complete X-ray crystallographic characterizations. J. Am. Chem. Soc. 2012;134:3139–3144. doi: 10.1021/ja210101f. [DOI] [PubMed] [Google Scholar]
- 54.Ceron MR, Li FF, Echegoyen L. An efficient method to separate Sc3N@C80Ih and D5h isomers and Sc3N@C78 by selective oxidation with acetylferrocenium [Fe(COCH3C5H4)Cp]+ Chem. Eur. J. 2013;19:7410–7415. doi: 10.1002/chem.201204219. [DOI] [PubMed] [Google Scholar]
- 55.Velde GT, et al. Chemistry with ADF. J. Comput. Chem. 2001;22:932–967. doi: 10.1002/jcc.1056. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data that support the findings of this study are available within the paper and its supplementary information files, and available from the corresponding author upon request. A data set collection of computational results is available from the online ioChem-BD repository and can be accessed via https://doi.org/10.19061/iochem-bd-2–16, where all intermediates described in Fig. 3 and Supplementary Fig. 10 can be found.