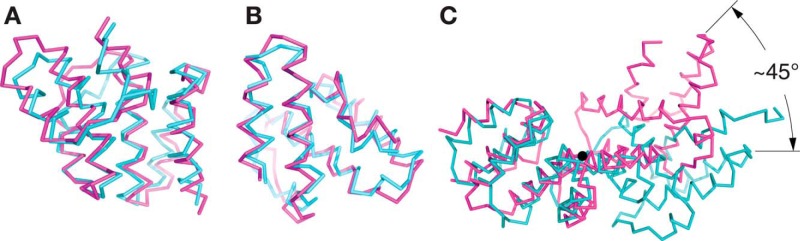
Figure 2.
The LpoA-N TPR-like domain has a typical superhelical twist in contrast to the EcLpoA-N NMR structure. The α-carbon traces of EcLpoA-N (PDB entry 2MYH) (19) are shown in cyan and HiLpoA-N in magenta. A, subdomain 1 of EcLpoA-N (residues 31–146) was superposed onto the corresponding region of HiLpoA-N. The RMSD of the Cα positions was calculated to be 1.6 Å for 94 of the 116 superposed residues selected by “super” in PyMOL. B, the latter portion of EcLpoA-N (residues 151–248) was superposed onto the corresponding region of the HiLpoA-N structure, with an RMSD = 1.7 Å for 98 of the 114 residues superposed. C, overlay of the HiLpoA-N (magenta) and the EcLpoA-N structures (cyan) after fitting their subdomains 1 as in A. The view is along the rotation axis (black circle) that relates the C-terminal portions (subdomains 2) of the two N-domain structures and passes through the center of helix H7 at residue 139 of HiLpoA-N.