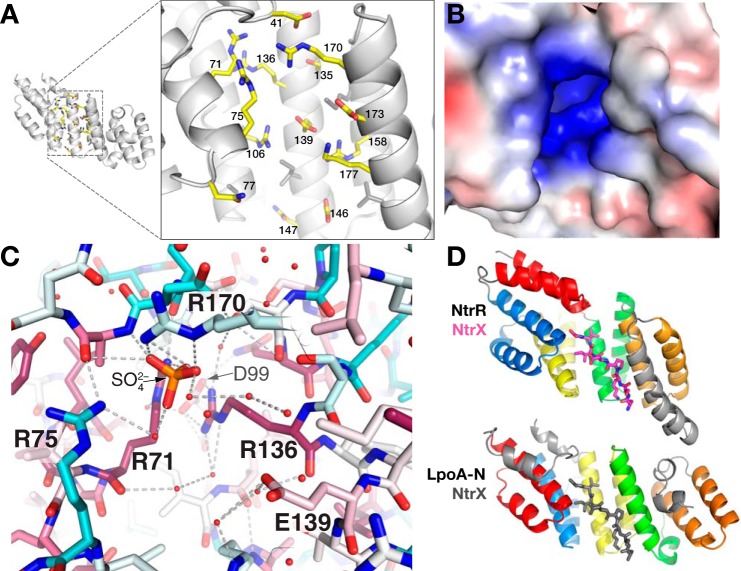
Figure 3.
Groove on the concave surface of LpoA-N. A, enlarged view of polar side chains that are exposed in the concave groove of the N domain. B, solvent contact surface of the N-domain groove, viewed as in A, and colored by electrostatic potential as calculated by APBS (20). The range of the potentials shown (red to blue) is −4 to 4 e−/Å3. C, sulfate (orange/red) bound in the groove of the LpoA-N structure and ligated directly by three Arg and through water to another Arg. Side-chain sticks are colored according to their degree of conservation calculated with sequences only selected from the Pasteurellaceae and Enterobacteriaceae families. D, the TPR-containing domain of NtrR (top, PDB entry 4GPK, residues 68–301) bound to its cognate peptide NtrX (magenta, residues 25–32) and LpoA-N (bottom). The first helix-turn-helix (red) motif of LpoA-N was oriented with the second helix-turn-helix motif (blue) of NtrR. For reference, the location of the NtrX peptide in the context of LpoA-N (in the groove) is shown in gray sticks.