Fig. 1.
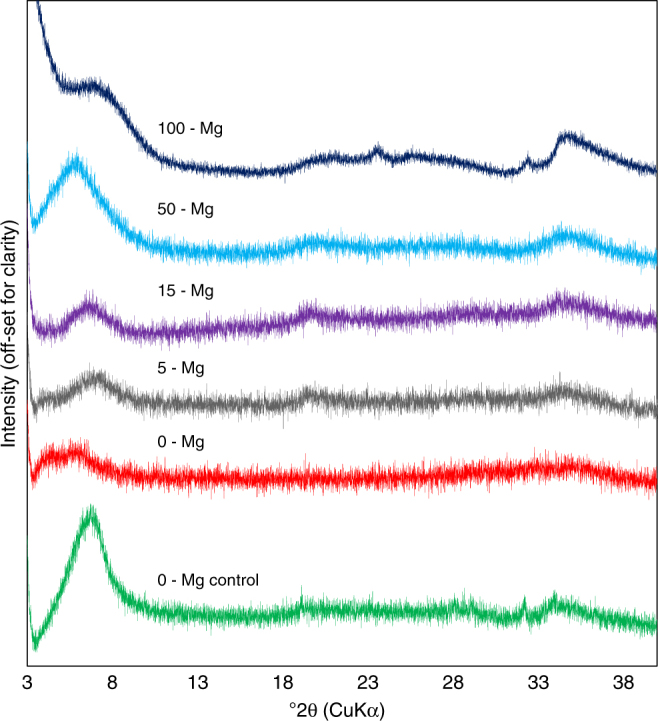
X-Ray diffractograms of the synthetic clay minerals. Samples were analyzed as oriented samples on Si-mounts. Note that the 001 diffraction of the 0-Mg nontronite control, using initial Fe2+, indicates that the control is crystalline, whereas the 0-Mg experiment, including only Fe3+ is largely amorphous. This is also shown in the 100 °C experiments (Supplementary Fig. 21) and is in agreement with the previous studies of Harder7, and Decarreau et al.8. Increasing Mg concentrations in the Fe3+ solutions resulted in increased crystallinity of the synthesized products. These results indicate that Fe-rich clay minerals can be precipitated under oxidized conditions, as long as Mg is present in at least small concentrations in the intial starting solution. The pure Mg precipitate had a much broader basal reflection, suggesting less coherent stacking along the c-axis
