Figure 5.
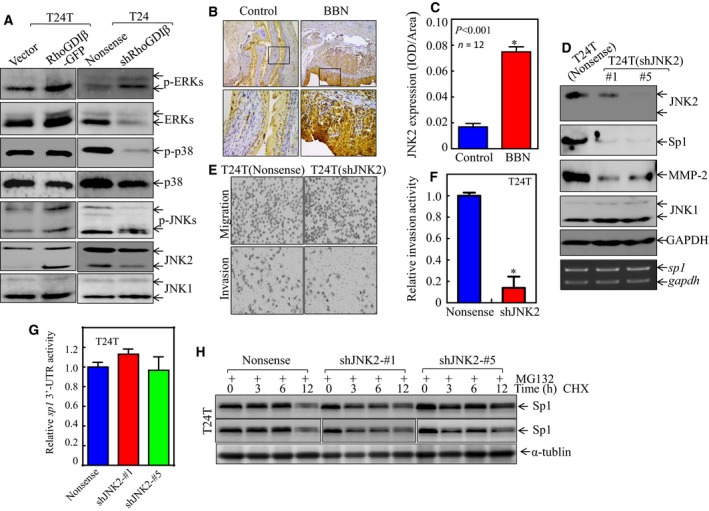
JNK2 specifically mediated Sp1 expression, in turn enhancing mmp‐2 expression and BC cell invasion. (A) The cell extracts obtained from the transfectants, as indicated, were subjected to western blot for determination of protein expression. (B,C) IHC‐P was carried out to evaluate JNK2 protein expression in mouse BC tissues as compared with normal bladder tissues. The optical density was analyzed and calculated, as described in Materials and Methods (n = 12). *Significant difference between the two groups of mice (P < 0.01). (D) The cell extracts obtained from the transfectants, as indicated, were subjected to western blot for determination of protein expression (top panel) or RT‐PCR for evaluation of sp1 mRNA expression (lower panel). (E,F) Invasive abilities of T24T(shJNK2) and T24T(Nonsense) cells were determined by using the BD BioCoat™Matrigel™ Invasion Chamber. The images was captured under inverted microscopy (E) and the relative invasion activity was plotted (F). The bars are mean ± SD from three independent experiments. *Significant difference between T24T(shJNK2) and T24T(Nonsense) cells (P < 0.05). (G) Wild‐type sp1 3′‐UTR‐drived luciferase reporters were co‐transfected together with pRL‐TK into T24T(Nonsense) and T24T(shJNK2) cells, respectively. Twenty‐four hours post‐transfection, the transfectants were extracted to evaluate the luciferase activity. The results were presented as sp1 3′‐UTR activity relative to the vector control transfectant, and each bar indicates mean ± SD from three independent experiments. (H) Sp1 protein stability was evaluated in both MG132 pretreated T24T(Nonsense) and T24T(shJNK2) cells in the presence of 50 μg·mL −1 cycloheximide (CHX). α‐Tubulin was used as a protein loading control.
