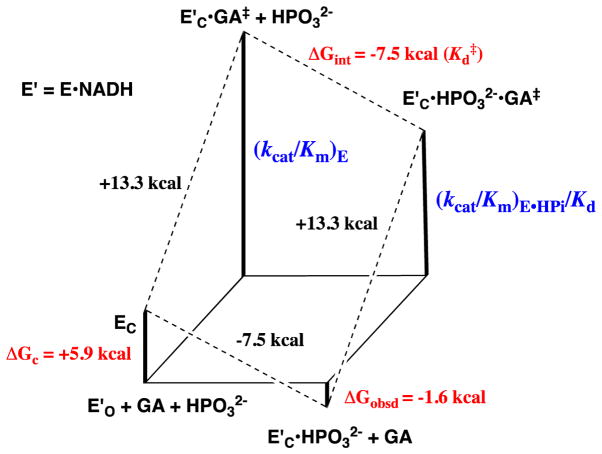
Figure 3.
Free energy diagram for GPDH-catalyzed reduction of glycolaldehyde (GA) by free GPDH, which exists mainly in the inactive open form (E′O, Scheme 3) and by GPDH that is saturated with phosphite dianion and exists in the active closed form (E′C•HPO32−). This diagram was constructed using kinetic parameters for Scheme 4 that were reported in Ref 28. The observed tighter binding of phosphite dianion to the transition state complex E′C•GA‡ to give EC•HPO32−•GA‡ (ΔGint = −7.5 kcal/mol) than to the free enzyme EO to give EC•HPO32− (ΔGobsd = −1.6 kcal/mol) is attributed the binding energy utilized to drive an unfavorable enzyme conformational change (ΔGC = +5.9 kcal/mol) that converts E′O to E′C (Scheme 3).