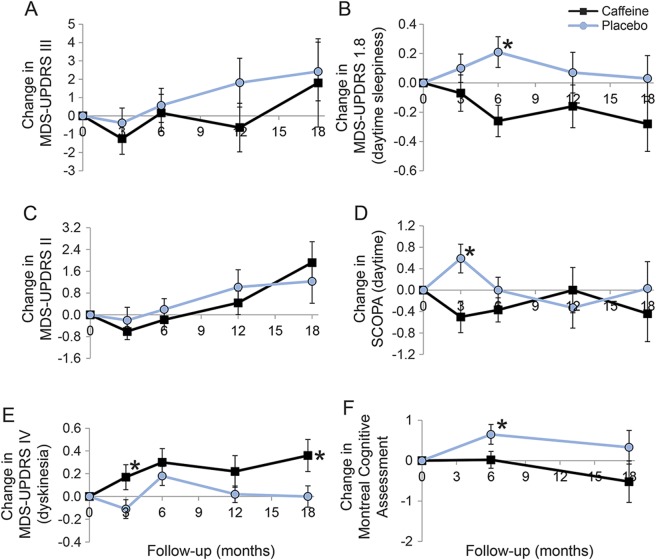
Figure 2. Change from baseline in selected Parkinson disease measures in caffeine vs placebo.
(A) Change in Movement Disorder Society–sponsored Unified Parkinson's Disease Rating Scale (MDS-UPDRS) III, (B) change in MDS-UPDRS 1.8 (daytime sleepiness), (C) change in MDS-UPDRS II, (D) change in Scales for Outcomes in PD (SCOPA) (daytime), (E) change in MDS-UPDRS IV (dyskinesia), (F) change in Montreal Cognitive Assessment. Note that the primary outcome is at 6 months; only a proportion of patients continued follow-up into 12 months (73%) and 18 months (55%). Error bars indicate standard error. The asterisk indicates a statistically significant difference between caffeine and placebo (p < 0.05).