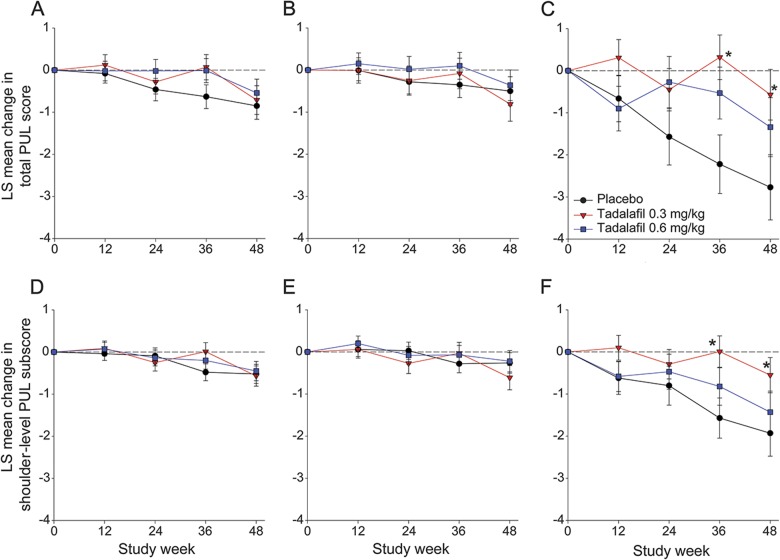
Figure 3. Mean change in total and shoulder level PUL score.
(A) Total PUL score in the total population (n = 114 in placebo, 99 in tadalafil 0.3 mg/kg, and 107 in tadalafil 0.6 mg/kg). (B) Total PUL score in patients ≤10 years of age (n = 95 in placebo, 68 in tadalafil 0.3 mg/kg, and 85 in tadalafil 0.6 mg/kg). (C) Total PUL score in patients >10 years of age (n = 19 in placebo, 31 in tadalafil 0.3 mg/kg, and 22 in tadalafil 0.6 mg/kg). (D) Shoulder-level PUL subscore in the total population (n = 116 in placebo, 101 in tadalafil 0.3 mg/kg, and 111 in tadalafil 0.6 mg/kg). (E) Shoulder-level PUL subscore in patients ≤10 years of age (n = 96 in placebo, 69 in tadalafil 0.3 mg/kg, and 89 in tadalafil 0.6 mg/kg). (F) Shoulder-level PUL subscore in patients >10 years of age (n = 20 in placebo, 32 in tadalafil 0.3 mg/kg, and 22 in tadalafil 0.6 mg/kg). Changes in total PUL score are from a total score range of 0 to 72; changes in shoulder-level PUL are from a total score range of 0 to 16. Higher score represents better function. Analyses for each efficacy parameter included all patients with nonmissing baseline and at least 1 nonmissing postbaseline value. *p < 0.05. LS = least squares; PUL = Performance of Upper Limb.