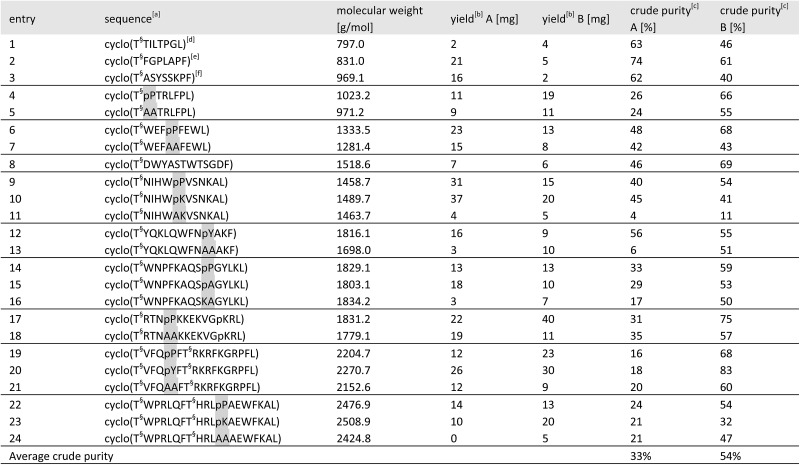
Table 2. Results of the cyclic peptide library synthesis by protected cyclization in solution (method A) and by KAHA cyclization (method B).
|
|
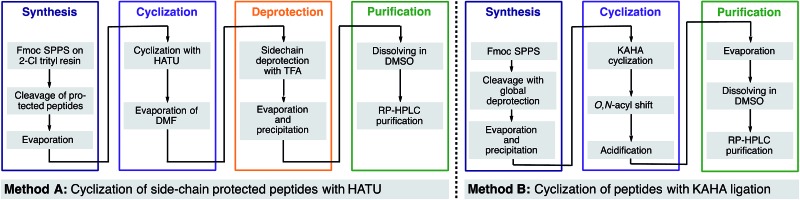
aHighlighted residues indicate the distinctive position of similar sequences with and without a turn inducing template. The last position indicates the α-ketoacid used for cyclization (Leu or Phe). The peptide concentration for cyclization was 6.25 mM for both methods. [T§ = (S)-homoserine].
bIsolated yields on 50 μmol scale after RP-HPLC purification.
cDetermined by HPLC as relative area% at 220 nm.
dGypsophin B25 analogue.
ePseudostellarin G25 analogue.
fSegetalin F25 analogue.
