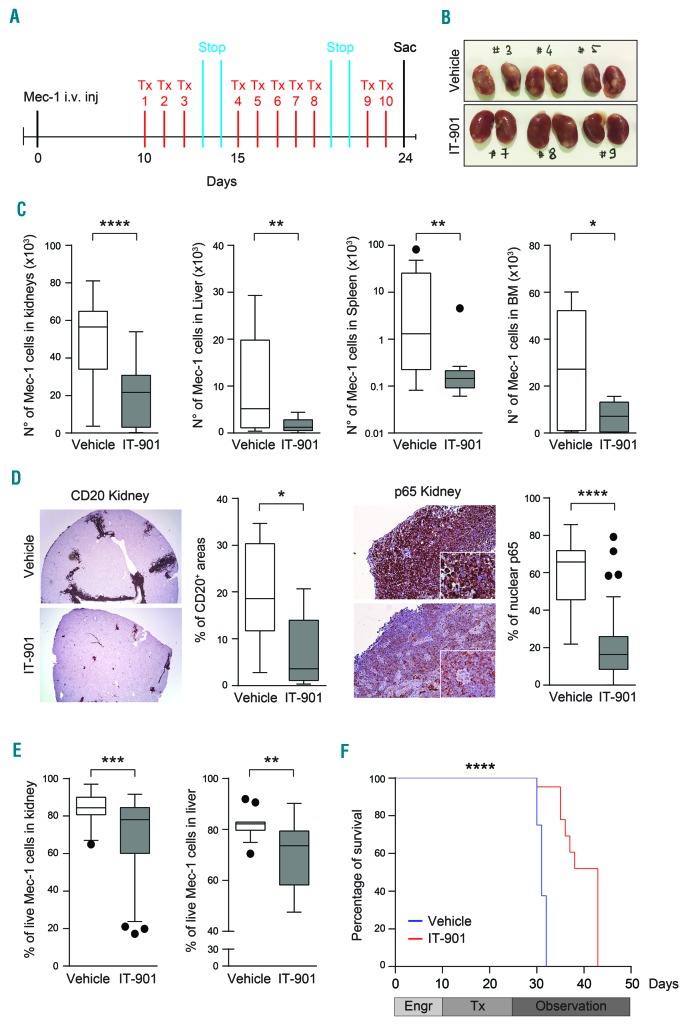
Figure 7.
IT-901 limits in vivo growth and spread of chronic lymphocytic leukemia (CLL) cells. (A) Representative scheme of the in vivo model. Mec-1 cells were intravenously injected in tail vein of NSG mice, left to engraft for ten days before starting the treatment with IT-901 or vehicle. (B) Images of kidneys obtained from vehicle- or IT-901-treated mice. (C) Mec-1 engraftment in different organs evaluated by flow cytometry after labeling of leukemic cells with anti-human-CD45 and -CD19 antibodies. Cumulative data of engraftment in kidneys, liver, spleen and bone marrow (BM) (n=8 different mice/group). (D) Immunohistochemical analyses and quantification of CD20 and nuclear p65 staining (reported as percentage of positive cells) in kidneys of vehicle- or IT-901-treated mice. (E) Viability of Mec-1 cells, purified from kidneys and liver of vehicle- or IT-901-treated mice, analyzed by flow cytometry (69% vs. 85% of viable cells in kidneys and 70% vs. 82% of viable cells in liver, respectively). (F) Kaplan-Meier curves showing survival of mice treated with IT-901 (n=8; red line; 43 days) compared to vehicle (n=8; blue line; 31 days). Engr: engraftment; Tx: treatment; Stop: drug holidays; Sac: euthanasia.