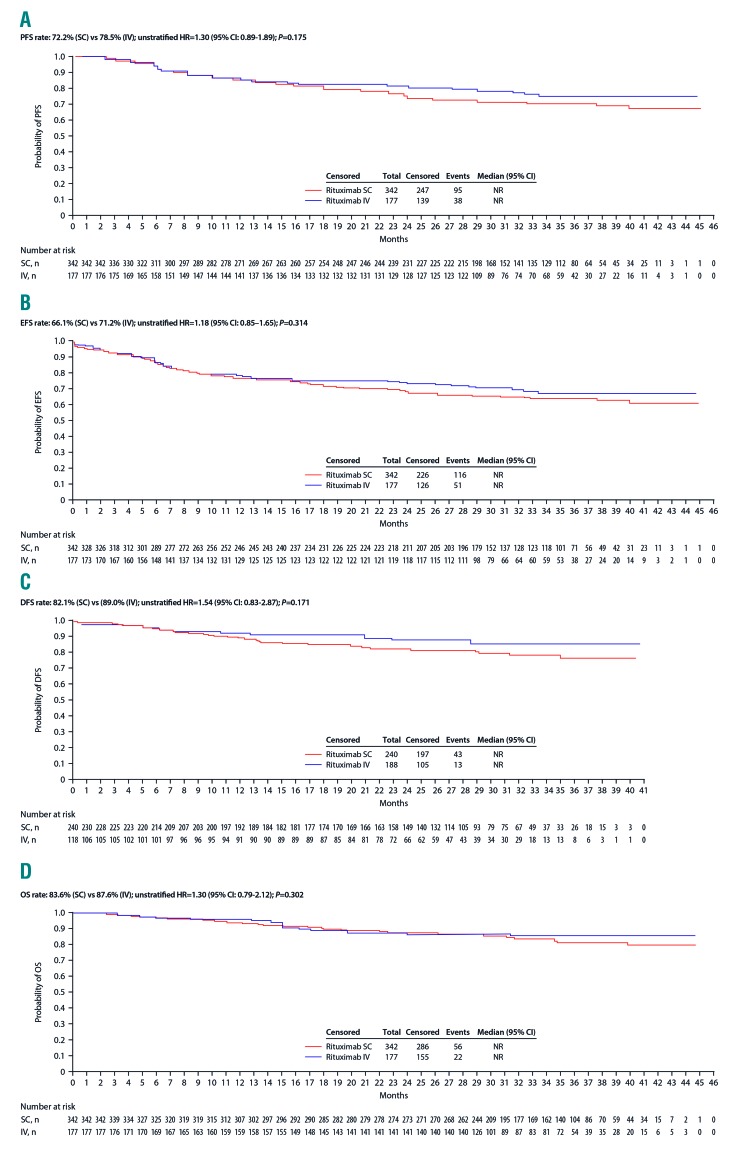
Figure 2.
Secondary time-to-event endpoints for rituximab SC and rituximab IV (intent-to-treat population). Analyses presented are (A) progression-free survival, (B) event-free survival, (C) disease-free survival, and (D) overall survival. CI: confidence interval; DFS: disease-free survival; EFS: event-free survival; HR: hazard ratio; IV: intravenous; OS: overall survival; PFS, progression-free survival; SC: subcutaneous.