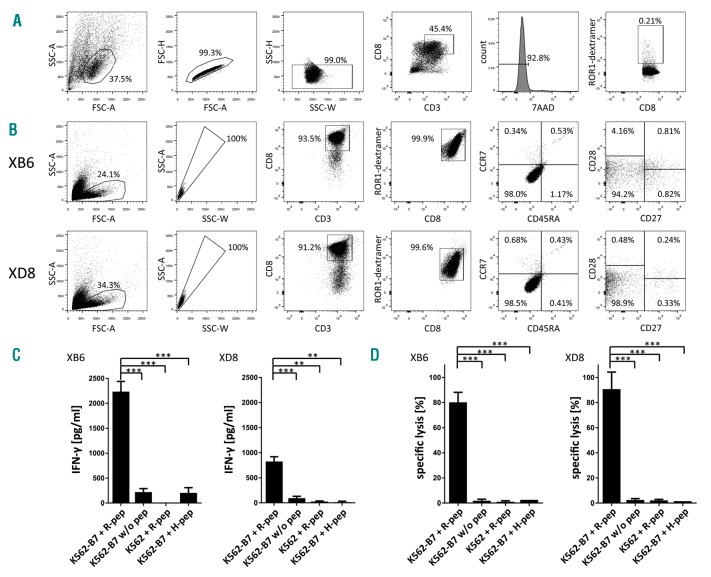
Figure 2.
Characterization of differentiation state and function of ROR1 peptide-specific T-cell clones XB6 and XD8. (A) Gating strategy used for the determination of the frequency of ROR1 peptide-specific CD8+ T cells in the polyclonal CD8+ T-cell population after stimulation with peptide-loaded moDCs is demonstrated. Doublets and dead cells (7-AAD positive) were excluded from the analysis. (B) Purity and differentiation state of the ROR1 peptide-specific CD8+ T-cell clones XB6 and XD8 are depicted. (C) ROR1 peptide-specific T-cell clones were co-incubated with ROR1 peptide-loaded, HLA-B*07:02-transduced K562 cells, unloaded HLA-B*07:02-transduced K562 cells, ROR1 peptide-pulsed, HLA-B*07:02− K562 cells, or HLA-B*07:02-transduced K562 cells loaded with the irrelevant peptide GPGHKARVL. After 24 h, supernatants were collected and IFN-γ concentration was determined by enzyme-linked immunosorbent assay (ELISA). The results are presented as mean ± s.d. of triplicate determinations. Statistical significance was calculated by the Student’s t-test. Asterisks indicate a statistically significant difference (*** P<0.001; ** P<0.01). (D) The T-cell clones XB6 and XD8 were co-cultured with 5 × 103 51Cr-labeled, ROR1 peptide-loaded, and HLA-B*07:02-transduced K562 cells per well at an E:T ratio of 10:1 for 4 h. Unloaded HLA-B*07:02-transduced K562 cells, ROR1 peptide-pulsed, HLA-B*07:02− K562 cells, or HLA-B*07:02-transduced K562 cells loaded with the irrelevant peptide GPGHKARVL served as controls. The results of the CD8+ T-cell clones are presented as mean ± s.d. of triplicate determinations. Statistical significance was calculated by the Student’s t-test. Asterisks indicate a statistically significant difference (*** P≤ 0.001).