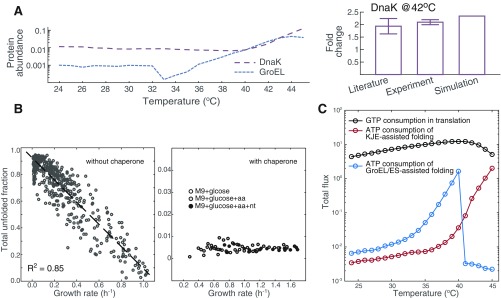
Fig. S1.
Model-predicted cellular cost related to the chaperone-assisted folding process. (A) Biosynthetic cost of the chaperones. Shown is the mass fraction of DnaK and GroEL in defined rich medium predicted by FoldME simulations. The calculated fold change of DnaK abundance at 42 °C compares well with measurements in the literature and in-house experiments. (B) Cost of the unfolded peptides. To show how the chaperone network controls the total unfolded protein fraction in the proteome, we simulated cell growth with and without the DnaK-assisted and GroEL/ES-assisted folding pathways. Without the chaperones, we simulated cell growth by sampling of each protein independently from the calculated distribution at 37 °C. The results showed an anticorrelation between growth rate and the mass fraction of the unfolded proteome. With chaperones functioning in the designed network, the total unfolded fraction is kept at a low level regardless of how the temperature changes the growth rate. (C) Energy cost of chaperone-assisted folding reactions, compared with that consumed by protein translation.