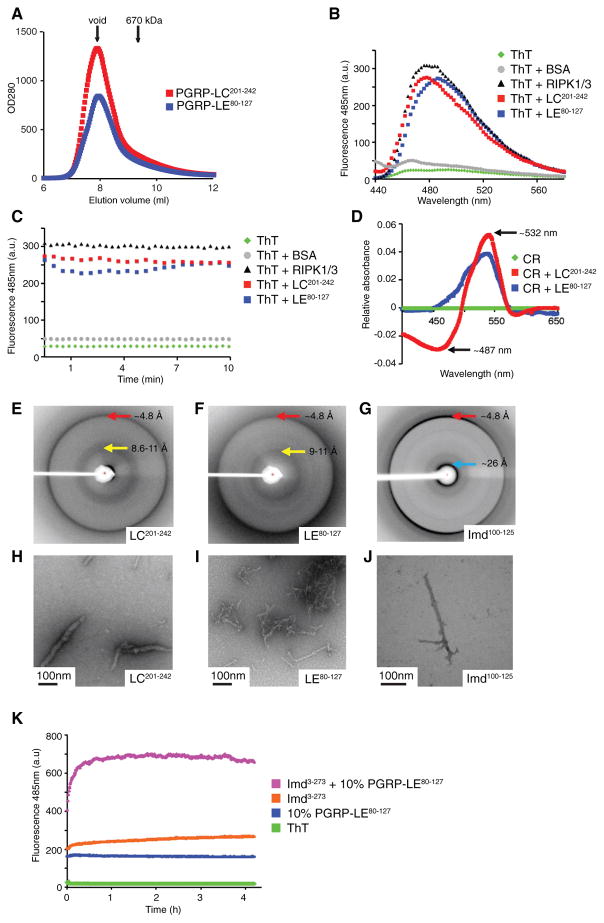
Figure 2. cRHIM Motifs of PGRP-LC, PGRP-LE, and Imd Form Amyloidal AggregatesIn Vitro.
(A) Superimposed gel filtration profiles of PGRP-LC and PGRP-LE cRHIM motifs. The Sumo-tagged fusion proteins eluted in void fractions.
(B–D) PGRP-LC and PGRP-LE cRHIMs bind am-yloid binding dyes Thioflavin T (ThT) and Congo Red (CR).
(B and C) Fluorescent emission spectra of ThT in the absence (green, no protein; gray, BSA) and presence (black, RIPK1/RIPK3; red, PGRP-LC201-242; blue, PGRP-LE80-127) of RHIM proteins.
(D) Absorption difference spectra of CR in the presence of PGRP-LC201-242 (red) or PGRP-LE80-127 (blue) compared to CR alone (green).
(E–J) PGRP-LC, PGRP-LE, and Imd filaments are classical amyloid fibrils. Scale bar: 100 nm.
(E–G) X-ray diffraction images of PGRP-LC (E), PGRP-LE (F), and Imd (G) cRHIM regions. The resolutions of dominant diffractions are labeled.
(H–J) Negative staining EM images of PGRP-LC (H), PGRP-LE (I), and Imd (J) cRHIM regions.
(K) ThT fluorescent emission spectra of Imd3-273 in the presence and absence of preformed PGRP-LE80-127 fibrils.