Abstract
Objective
The purpose of this study was to perform a systematic review and meta-analysis to evaluate the effect of high-dose versus low-dose haemofiltration on the survival of critically ill patients with acute kidney injury (AKI). We hypothesised that high-dose treatments are not associated with a higher risk of mortality.
Design
Meta-analysis.
Setting
Randomised controlled trials and two-arm prospective and retrospective studies were included.
Participants
Critically ill patients with AKI.
Interventions
Continuous renal replacement therapy.
Primary and secondary outcome measures
Primary outcomes: 90-day mortality, intensive care unit (ICU) mortality, hospital mortality; secondary outcomes: length of ICU and hospital stay.
Result
Eight studies including 2970 patients were included in the analysis. Pooled results showed no significant difference in the 90-mortality rate between patients treated with high-dose or low-dose haemofiltration (pooled OR=0.90, 95% CI 0.73 to 1.11, p=0.32). Findings were similar for ICU (pooled OR=1.12, 95% CI 0.94 to 1.34, p=0.21) and hospital mortality (pooled OR=1.03, 95% CI 0.81 to 1.30, p=0.84). Length of ICU and hospital stay were similar between high-dose and low-dose groups. Pooled results are not overly influenced by any one study, different cut-off points of prescribed dose or different cut-off points of delivered dose. Meta-regression analysis indicated that the results were not affected by the percentage of patients with sepsis or septic shock.
Conclusion
High-dose and low-dose haemofiltration produce similar outcomes with respect to mortality and length of ICU and hospital stay in critically ill patients with AKI.
This study was not registered at the time the data were collected and analysed. It has since been registered on 17 February 2017 at http://www.researchregistry.com/, registration number: reviewregistry211.
Keywords: acute kidney injury, dose, intensive care unit, intensity, renal dialysis, renal-replacement therapy
Strengths and limitations of this study.
The strengths of this study are the inclusion of the most current literature and the meta-regression analysis that evaluated the impact of patients with sepsis or septic shock on the overall pooled analysis.
The limitation of this analysis is the considerable variation across the studies with regard to the prescribed doses for the high-dose and low-dose haemofiltration.
Introduction
Acute kidney injury (AKI) occurs in at least 5% of patients with who are admitted to the intensive care unit (ICU) and is an independent predictor of mortality.1–3 In addition, about 50% of patients with septic shock will experience AKI.4 The prognosis of patients with AKI is low, with a mortality rate of up to 70% despite improvements in haemodialysis and availability.5–7
Two methods for obtaining clearance in patients with AKI who require renal support are haemofiltration and haemodialysis. Haemofiltration uses convection to aid in the removal of middle molecular weight solutes, which is determined by the pore size of the membrane.8 Haemofiltration is believed to be superior to haemodialysis in patients with AKI as it is thought it can remove the toxic mediators of sepsis and inflammation.8
For patients who require renal replacement therapy (RRT), the treatment dose or intensity may influence outcomes. Continuous renal replacement therapy (CRRT) in the form of haemofiltration is an option for treating patients with AKI and may provide better clearance of toxic molecules, acid–base homeostasis and removal of inflammatory mediators that can contribute to organ injury and dysfunction than other methods.9–13 However, the optimum dosage of haemofiltration, including the ideal timing and dose is not clear. Some studies have reported benefits with intensive doses of CRRT with respect to mortality,14 15 while others have not.16–19
Several prior systematic reviews and meta-analyses have assessed the use of CRRT for treating AKI. These studies found that high-dose CRRT was not associated with a decrease in mortality in patients with AKI.20–22 Since the publication of these reviews, additional clinical studies have been published that addressed the use of CRRT in AKI.23 24 Prior reviews have addressed both haemofiltration and haemodialysis, which may not provide sufficient data with respect to either method.
Thus, the purpose of this study was to perform a systematic review and meta-analysis to evaluate the effect of high-dose versus low-dose haemofiltration on the survival of critically ill patients with AKI. We hypothesised that high-dose treatments are not associated with a higher risk of mortality.
Materials and methods
Search strategy
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. This study was not registered at the time the data were collected and analysed. It has since been registered on 17 February 2017 at http://www.researchregistry.com/, registration number: reviewregistry211.
PubMed, Medline, Cochrane and Google Scholar databases were searched until 22 June 2016 using the following search terms: renal replacement therapy, renal dialysis, acute kidney injury, intensive care unit, intensity and dose. Included studies were randomised controlled trials (RCTs), two-arm prospective, retrospective or cohort studies that evaluated critically ill patients with AKI who received haemofiltration. Included studies had to report quantitative outcomes of interest. Letters, comments, editorials, case reports, proceeding and personal communications were excluded. Studies that evaluated patients who had received previous RRT during the same hospital admission or who were on maintenance dialysis for end-stage kidney disease were excluded. The database searches were performed by two independent (two of the authors) reviewers. The authors independently reviewed all potential studies and extracted data of interest. A third reviewer was consulted to resolve any questions regarding inclusion of studies or data in the analysis, and a decision was arrived at by consensus.
Data extraction and quality assessment
The following information/data were extracted from studies that met the inclusion criteria: the name of the first author, year of publication, study design, number of participants in each group, participants’ age and gender and the major outcomes of death up to 90 days (90-day mortality), ICU mortality, hospital mortality and length of hospital or ICU stay.
The quality of the included studies was evaluated using the Cochran Risk of Bias tool outlined in the Cochrane Collaboration Handbook for Systematic Reviews of Interventions V.5.1.0.25
Statistical analysis
Primary outcomes were 90-day mortality, ICU mortality and hospital mortality. Secondary outcomes were length of ICU and hospital stay. Comparisons of the different mortality rates between patients receiving high-dose or low-dose haemofiltration were presented by OR and 95% CI; an OR >1 indicated that patients treated with high-intensity haemofiltration had a higher risk of death. The effect size of length of ICU and hospital stay was reported as difference in means; a difference in means >0 indicated longer ICU or hospital stay in patients treated with high-dose haemofiltration. Pooled estimates for ORs and difference in means were calculated using the DerSimonian and Laird random-effects model. A two-sided p value <0.05 was considered statistically significant.
Heterogeneity was assessed using the Cochran Q and the I2 statistic. For the Q statistic, p<0.10 was considered statistically significant for heterogeneity. The I2 statistic indicates the percentage of the observed between-study variability due to heterogeneity. The suggested ranges are as follows: no heterogeneity (I2=0% to 25%), moderate heterogeneity (I2=25% to 50%), large heterogeneity (I2=50% to 75%) and extreme heterogeneity (I2=75% to 100%).
Sensitivity analysis was performed for the primary outcomes using the leave-one-out approach. Due to various definitions of high-dose and low-dose haemofiltration between the studies, additional sensitivity analysis was performed to examine the stability of pooled estimates according to various cut-off points of prescribed dose as well as the actual delivered dose. Meta-regression analysis was performed to examine whether the percentage of patients with sepsis or septic shock influenced the pooled estimates of the associations between haemofiltration and outcomes of interest. All analyses were performed using Comprehensive Meta-Analysis statistical software, V.2.0 (Biostat, Englewood, New Jersey, USA).
Results
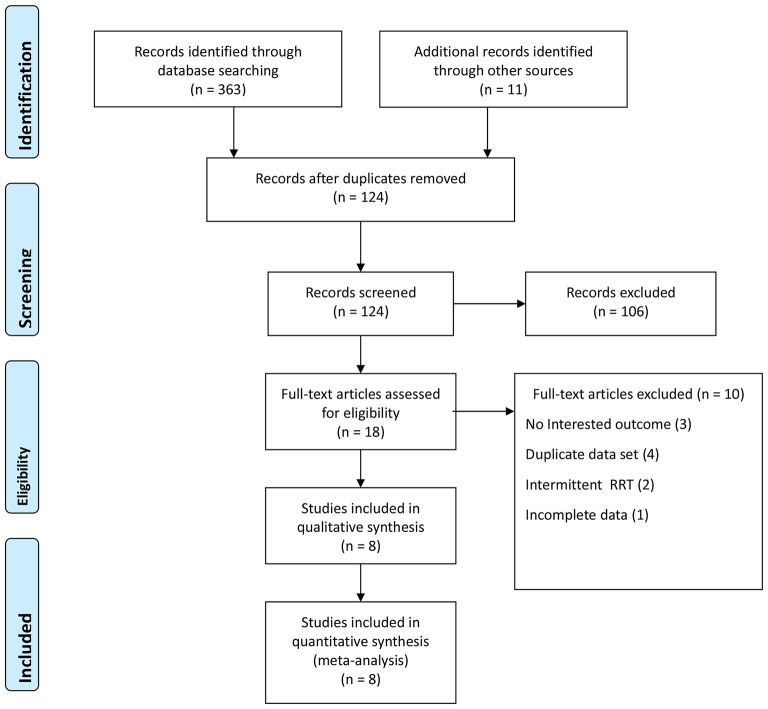
A total of 374 studies were identified in the initial search, of which 250 were excluded for being duplicate publications (figure 1). Of the remaining 124 studies, 106 were excluded for not being relevant by review of title and/or abstract. The remaining 18 full-text articles were examined, and 10 were excluded, the reasons for which are shown in figure 1. Thus, eight studies were included in the meta-analysis.14 16 17 19 23 24 26 27
Figure 1.
PRISMA flow diagram of study selection.
Of the eight studies, seven were RCTs14 16 17 23 24 26 and one was a prospective study27 (table 1). A total of 2970 patients were included, and the number of patients per study ranged from 19 to 1465. The mean patient age ranged from 59 to 73 years, over half of the patients were male (54% to 80%%), and the causes for of AKI requiring RRT were sepsis, surgery (including cardiovascular surgery) and septic shock. Six of the eight studies reported mean Acute Physiology and Chronic Health Evaluation (APACHE) II or III scores for the groups studied, and the mean scores were similar between the groups in the individual studies. The type of treatment and the definition of high-dose or more-intensive therapy varied across the studies. The doses used also varied, with low-dose ranging from 20 to 36 mL/kg/h and high-dose ranging from 35 to 85 mL/kg/h.
Table 1.
Summary of basic characteristics of studies included in the meta-analysis
| Study | Study design | Number of patients | Treatment | Prescribed dose (mL/kg/h) | Delivered dose (mL/kg/h) | Duration (d) | Age (year) | Male (%) | Major cause of AKI | Sepsis | Oliguria | Mean APACHE II score |
| Joannes-Boyau et al23 | RCT | 66 | High-volume HF | 70 | 65.6 (40–67.9)* | 96 hours | 68 (58–77)* | 68.0 | Sepsis | 66 (100%) | ||
| 71 | Standard-volume HF | 35 | 33.2 28.7–33.6)* | 70 (58–75)* | 54.0 | 71 (100%) | ||||||
| Vesconi et al27 | Prospective | 75 | More intensive | 35 | 44.8 (9.4) | 2 (1–3)* | 61.01 (17.4) | 58.1 | Surgery | 33 (40.5%) | 36 (48.6%) | |
| 202 | Less intensive | 21–34 | 26.9 (4.0) | 4 (2–8)* | 63.48 (15.9) | 67.8 | 81 (40.1%) | 84 (41.7%) | ||||
| 61 | Less intensive | 20 | 15.4 (4.2) | 3 (2–6)* | 59.05 (19.0) | 73.8 | 19 (31.2%) | 30 (49.5%) | ||||
| Zhang et al24 | RCT | 141 | EHVHF | 85 | 87.54 (12.54) | 9.38 (12. 06) | 56.62 (16.38) | 58.9 | Septic shock | 72 (51.06%) | 21.97 | |
| 139 | HVHF | 50 | 49.99 (9.65) | 8.88 (10.79) | 59.96 (18.81) | 64.0 | 69 (49.64%) | 22.6 | ||||
| Bouman et al16 | RCT | 35 | EHV | 72 | 48.2 (42.3–58.7)* | 68.5 (28.0–140.8)*,† | 68 (13) | 60.0 | Cardiac surgery | 35 (100%) | 23.5 | |
| 35 | ELV | 24–36 | 20.1 (17.5–22.0)* | 94.0 (53.0–181.5)*,† | 70 (10) | 57.0 | 35 (100%) | 21.7 | ||||
| 36 | LLV | 24–36 | 19.0 (16.6–21.2)* | 69.5 (28.3–157.7)*,† | 67 (13) | 61.0 | 30 (100%) | 23.6 | ||||
| Tolwani et al19 | RCT | 100 | High dosage | 35 | 29 | 10.0 (9.8) | 58 (16) | 59.0 | Septic shock | 54 (54%) | 64 (64%) | 26 |
| 100 | Standard dosage | 20 | 17 | 9.7 (11.3) | 62 (15) | 57.0 | 54 (54%) | 63 (63%) | 26 | |||
| Bellomo (2009) | RCT | 722 | Higher-Intensity CRRT | 40 | 33.4 (12.8) | 6.3 (8.7) | 64.7 (14.5) | 65.7 | Sepsis | 360 (49.9) | 430 (59.6%) | 102.5† |
| 743 | Lower-Intensity CRRT | 25 | 22 (17.8) | 5.9 (7.7) | 64.4 (15.3) | 63.5 | 363 (48.9) | 444 (59.8%) | 102.3† | |||
| Boussekey et al26 | RCT | 9 | HVHF | 65 | 62 | 7 (2–17)* | 68 (58–74)* | 78.0 | Sepsis | 9 (100 %) | 31 | |
| 10 | LVHF | 35 | 32 | 6 (2–14)* | 72.5 (54–77)* | 80.0 | 10 (100%) | 33.5 | ||||
| Ronco et al14 | RCT | 146 | 20 | 61 (10) | 55.5 | Surgery | 20 (14%) | 22 | ||||
| 139 | 35 | 59 (9) | 55.4 | 17 (12%) | 24 | |||||||
| 140 | 45 | 63 (12) | 57.1 | 15 (11%) | 22 |
*Data were presented by median and IQR and by mean and SD if not specified.
†Measured by APACHE III.
‡Numbers were shown in hours.
AKI, acute kidney injury; CRRT, continuous renal-replacement therapy; EHV, early high-volume haemofiltration; EHVHF, extra high-volume haemofiltration; ELV, early low-volume haemofiltration; HF, haemofiltration; HVHF, high-volume hemofiltration; LLV, late low-volume haemofiltration; LVHF, low-volume haemofiltration; RCT, randomised controlled trial.
Meta-analysis
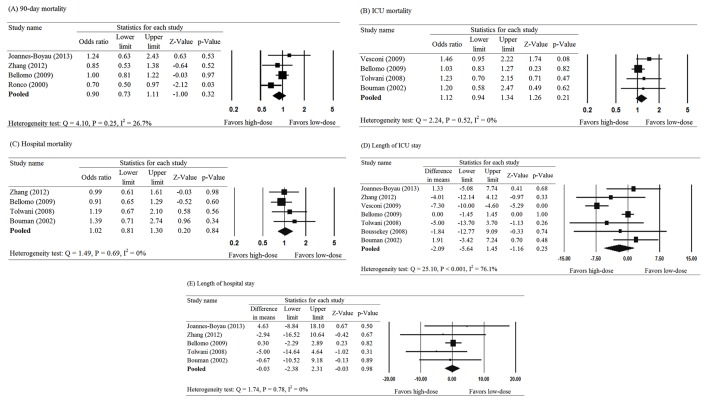
Results of meta-analysis of the primary outcomes of within 90-day morality,14 17 23 24 ICU mortality16 17 19 27 and hospital mortality16 17 19 24 are presented in figure 2. The pooled results showed no significant difference in the 90-mortality rate between patients treated with high-dose or low-dose haemofiltration (pooled OR=0.90, 95% CI 0.73 to 1.11, p=0.32) (figure 2A). The findings were similar for ICU mortality (pooled OR=1.12, 95% CI 0.94 to 1.34, p=0.21) and hospital mortality (pooled OR=1.02, 95% CI 0.81 to 1.30, p=0.84) (figure 2B, C). There was low to moderate heterogeneity across the studies for each outcome (Q=4.10, p=0.25, I2=26.7%; Q=2.24, p=0.52, I2=0%; and Q=1.49, p=0.69, I2=0%, respectively).
Figure 2.
Meta-analysis for treatment effect of haemofiltration on (A) mortality within 90 days, (B) ICU mortality, (C) in hospital mortality, (D) length of ICU stay and (E) length of hospital stay. ICU, intensive care unit.
No significant difference was found in the length of ICU stay between patients who received high- versus low-dose treatment (pooled difference in means=−2.09, 95% CI −5.64 to 1.45, p=0.25) (figure 2D). However, large heterogeneity was observed across the seven studies that reported data for length of ICU stay (Q=25.10, p<0.001, I2=76.1%). The results were similar for length of hospital stay (pooled difference in means=−0.03, 95% CI −2.38 to 2.31, p=0.98); however, no heterogeneity was observed for data regarding length of hospital stay (Q=1.74, p=0.78, I2=0%) (figure 2E).
Sensitivity analysis, meta-regression analysis and quality assessment
Sensitivity analysis was performed in several ways. First, we used the leave-one-out approach to examine whether any single study influenced the pooled results of primary outcomes. The pooled results for the three primary outcomes did not significantly change when each study was removed in turn (figure 3). Second, the use of various cut-off points of prescribed dose might minimise the influence of the various definitions of high-dose and low-dose in the included studies. Analysis indicated that the results were stable regardless of cut-off points of prescribed dose. Furthermore, we also performed analyses for the actual delivered dose with the same cut-off points, and the statistical significance was consistent when delivered dose was used in the analysis (table 2). Taken together, these findings indicate that the pooled results are not overly influenced by any one study, different cut-off points of prescribed dose or different cut-off points of delivered dose.
Figure 3.
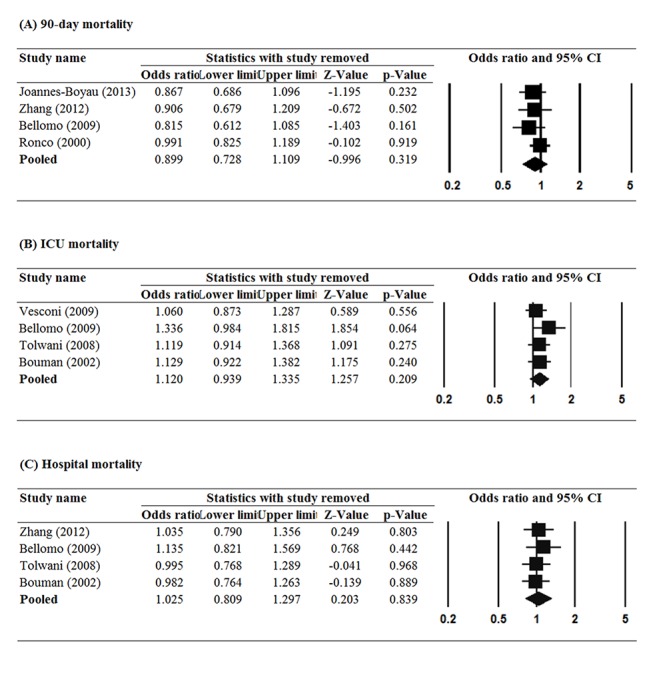
Sensitivity analysis using leave-one-out approach for the treatment effect of haemofiltration (A) mortality within 90 days, (B) ICU mortality and (C) in hospital mortality.
Table 2.
Sensitivity analysis for treatment effect on mortality according to different cut-off points of prescribed dose and delivered dose
| Number of studies included | Pooled OR | Lower limit | Upper limit | Z value | p Value | |
| Prescribed dose | ||||||
| (A) 90-day mortality | ||||||
| 50 mL/kg/h | 2 | 0.97 | 0.66 | 1.43 | −0.16 | 0.88 |
| 40 mL/kg/h | 3 | 0.83 | 0.50 | 1.38 | −0.71 | 0.48 |
| 30 mL/kg/h | 2 | 0.73 | 0.39 | 1.38 | −0.97 | 0.33 |
| (B) ICU mortality | ||||||
| 50 mL/kg/h | 1 | 1.20 | 0.58 | 2.47 | 0.50 | 0.62 |
| 40 mL/kg/h | 2 | 1.04 | 0.85 | 1.28 | 0.37 | 0.72 |
| 30 mL/kg/h | 3 | 1.13 | 0.92 | 1.38 | 1.18 | 0.24 |
| (C) Hospital mortality | ||||||
| 50 mL/kg/h | 2 | 1.11 | 0.75 | 1.65 | 0.53 | 0.60 |
| 40 mL/kg/h | 2 | 1.02 | 0.71 | 1.45 | 0.08 | 0.94 |
| 30 mL/kg/h | 2 | 0.98 | 0.73 | 1.31 | −0.15 | 0.88 |
| Delivered dose | ||||||
| (A) 90-day mortality | ||||||
| 50 mL/kg/h | 2 | 0.97 | 0.66 | 1.43 | −0.16 | 0.88 |
| 40 mL/kg/h | 1 | 1.24 | 0.63 | 2.43 | 0.63 | 0.53 |
| 30 mL/kg/h* | 1 | 1.00 | 0.81 | 1.23 | −0.03 | 0.97 |
| (B) ICU mortality | ||||||
| 50 mL/kg/h | 1 | 1.20 | 0.58 | 2.47 | 0.50 | 0.62 |
| 40 mL/kg/h | 2 | 1.39 | 0.96 | 2.00 | 1.75 | 0.08 |
| 30 mL/kg/h | 2 | 1.16 | 0.84 | 1.62 | 0.90 | 0.37 |
| (C) Hospital mortality | ||||||
| 50 mL/kg/h | 1 | 0.99 | 0.61 | 1.61 | −0.03 | 0.98 |
| 40 mL/kg/h | 1 | 1.39 | 0.71 | 2.74 | 0.96 | 0.34 |
| 30 mL/kg/h | 1 | 0.91 | 0.65 | 1.29 | −0.52 | 0.60 |
*Ronco et al14 did not provide information on delivered dose of continuous renal replacement therapy and therefore was excluded.
ICU, intensive care unit.
Meta-regression analysis was performed to examine whether patients with sepsis or septic shock affected the overall pooled analysis. The reason for the analysis was based on the fact that sepsis and septic shock differ in terms of blood pressure instability and possible emergent death. The results showed that the regression coefficients had a slope close to 0, indicating that the associations between RRT and selected outcomes were not influenced by the percentage of patients with sepsis or septic shock (p values for all slope coefficients >0.05) (table 3).
Table 3.
Meta-regression analysis for each outcome
| Outcome | Intercept* | Slope* |
| Mortality within 90 days | −0.42 (0.19) | 0.01 (0.004) |
| ICU mortality | 0.31 (0.35) | −0.004 (0.01) |
| Hospital mortality | 0.31 (0.34) | −0.01 (0.01) |
| Length of ICU stay | −2.89 (1.90) | 0.03 (0.04) |
| Length of hospital stay | −1.74 (4.13) | 0.034 (0.08) |
*Presented as point estimate of coefficient and SE.
ICU, intensive care unit.
Assessment of the quality of the included studies using the Cochran Risk of Bias tool indicated that there was low risk of bias for most of the studies (Figure S1A and S1B). One exception was the study of Vesconi et al,27 which showed a high risk of selection, performance and detection bias (Figure S1B). Overall, the included studies were of adequate quality.
bmjopen-2016-014171supp001.pdf (213.2KB, pdf)
Publication bias assessment was not performed due to limited number of included studies; 10 or more studies are necessary to assess publication bias.28
Discussion
The purpose of this study was to conduct a systematic review and meta-analysis to examine the effect of haemofiltration dosage on mortality, length of hospital stay, and length of ICU stay in patients with AKI. For all outcomes examined, there was no difference between patients who received high- versus low-dose haemofiltration. The results were consistent when the analyses used prescribed or delivered dose, and not influenced by the percentage of patients with sepsis or septic shock. Sensitivity analysis and quality assessment indicated no one study dominated the results, and that the included data were of adequate quality.
The results of this study are consistent with three prior meta-analyses, all of which found no survival benefit, or increased mortality, of high-dose CRRT in patients with acute renal failure.20–22 Although, our findings are similar to prior studies, a strength of our analysis is the meta-regression analysis with evaluated the impact of patients with sepsis or septic shock on the overall pooled analysis. The meta-regression analysis indicated that heterogeneity due to a mixed population of sepsis and septic shock patients did not influence the pooled results. In addition, we included two additional studies that were not included in the prior meta-analyses. The consistency of finding across the different meta-analyses, and findings of our meta-regression analysis, suggest that the delivered dose is not affected by the presence of systemic infection, and any variance seen when treating patients may reflect the severity of the acute kidney disease and/or an individual patient’s condition.
The data from clinical studies on the benefit of high-dose haemofiltration in critically ill patients have been inconsistent. In 2000, Ronco et al14 reported improved survival with higher total effluent volumes (>45 mL/kg/h) in patients with septic AKI, and in 2008, in a small pilot study, Boussekey et al26 found that high-dose RRT was associated with an improved haemodynamic profile. However, that study did not find any significant effect on survival or organ dysfunction. In contrast, two randomised controlled multicentre studies found no added survival benefit of high-dose compared with standard-dose CRRT in critically ill patients with AKI.17 18 A more recent study by Joannes-Boyau et al23 also found no evidence that high-dose (70 mL/kg/h) compared with standard-dose (35 mL/kg/h) RRT resulted in reduction in 28-day mortality, or to early improvements in haemodynamic profile or organ dysfunction in septic shock patients with AKI.23
A prior meta-analysis compared the efficacy of extended daily dialysis (EDD) and CCRT in treating patients with AKI. Zhang et al29 included 17 studies from 2000 to 2014 with a total of 1208 patients. Meta-analysis of the included RCTs (n=10) found no difference in mortality rates between EDD and CRRT (relative risk, 0.90; 95% CI 0.74 to 1.11; p=0.3). However, lower mortality risk was observed with EDD compared with CRRT in observational studies (relative risk, 0.86; 95% CI 0.74 to 1.00; p=0.05). For both RCTs and observational studies, recovery of kidney function, fluid removal and days in the ICU were similar between procedures. The authors concluded that the findings from RCTs suggest that CRRT and EDD have similar efficacy and that the difference in mortality observed in the analysis of the observational studies may be confounded by selection bias. The potential confounding effect of observational studies is also indicated by our quality assessment of the included studies that indicated that the observational study of Vesconi et al27 had a high risk of selection bias, as well as performance and detection bias.
The current analysis focused on haemofiltration. However, haemodialysis is also used to treat patients with AKI. Friedrich et al30 performed a meta-analysis in 2012 that included 19 RCTs that focused on the difference between haemofiltration and haemodialysis with similar doses. They found weak evidence supporting the increased clearance of medium to large molecules by haemofiltration compared with haemodialysis, but there was no difference in mortality between the two methods. No dose comparison was performed in their study.
Our study was limited by the considerable variation across the studies with regard to the prescribed doses for high-dose and low-dose haemofiltration. It is difficult to standardise the prescribed and delivered doses across the studies due to differences in equipment used and personnel. There was also a wide range of effect size, and several of the studies reported opposite findings. In addition, only four of the included studies reported data for the primary endpoint of mortality within 90 days, and not all the studies were RCTs. In addition, due to the heterogeneity of dosing across studies, the differences in the definition of high-dose, and the fact that the raw data for each group was not presented, it was difficult to group the analysis according to a cut-off value of the standard of care dose of 35 mL/kg/h. For example, Vesconi et al27 defined 35 mL/kg/h as ‘more intensive’ and compared the finding of that dosing with ‘less intensive’ <20 to 34 mL/kg/h. This differed, for example, from the study by Zhang et al,29 which compared 85 mL/kg/h with 50 mL/kg/h. It is highly possible that this variability may have confounded our results. However, sensitivity analysis found that no one study overly influences the findings; thus, suggesting that although heterogeneity in dosing was present, the pooled results are robust. In addition, six of the eight studies reported mean APACHE II or III scores for the groups studied, and the mean scores were similar between the groups in the individual studies, indicating that the illness severity was similar between the groups in each of these six studies. Subgroup analyses of different covariates, such as according to renal function, would aid in the analysis; however, due to limited availability of raw data, few variables can be investigated. Lastly, our original intention was to perform a meta-analysis examining the outcomes of using different doses of RRT, and during our initial literature search, we included all modalities of CRRT. However, we found that the majority of studies that compared different dosages used haemofiltration rather than other modalities. For this reason, we limited the analysis to haemofiltration.
The results of this meta-analysis found that mortality rates and length of ICU and hospital stay were not different between critically ill patients with AKI who received high-dose or low-dose haemofiltration.
Supplementary Material
Acknowledgments
We would like to express our sincere gratitude to our colleagues whose names did not appear as authors but also contributed to the study. We especially thank Xiao Tan, Jifu Jin and Zhouping Zou for collecting the data.
Footnotes
Contributors: PL: study design; literature research; manuscript preparation. LQ: study design, literature research and manuscript preparation. DQ: manuscript preparation, data acquisition and data analysis. BS: manuscript preparation, data acquisition and data analysis. YW: manuscript preparation, data acquisition and data analysis. JX: manuscript preparation, data acquisition and data analysis. WJ: data analysis and statistical analysis. HZ: data analysis and statistical analysis. XD: guarantor of integrity of the entire study and study concepts. JT: study concepts, study design, guarantor of integrity of the entire study and manuscript editing.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Metnitz PG, Krenn CG, Steltzer H, et al. . Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med 2002;30:2051–8. 10.1097/00003246-200209000-00016 [DOI] [PubMed] [Google Scholar]
- 2. Uchino S, Kellum JA, Bellomo R, et al. . Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 2005;294:813–8. 10.1001/jama.294.7.813 [DOI] [PubMed] [Google Scholar]
- 3. Uchino S, Bellomo R, Goldsmith D, et al. . An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med 2006;34:1913–7. 10.1097/01.CCM.0000224227.70642.4F [DOI] [PubMed] [Google Scholar]
- 4. Bagshaw SM, Uchino S, Bellomo R, et al. . Timing of renal replacement therapy and clinical outcomes in critically ill patients with severe acute kidney injury. J Crit Care 2009;24:129–40. 10.1016/j.jcrc.2007.12.017 [DOI] [PubMed] [Google Scholar]
- 5. Keir I, Kellum JA. Acute kidney injury in severe sepsis: pathophysiology, diagnosis, and treatment recommendations. J Vet Emerg Crit Care 2015;25:200–9. 10.1111/vec.12297 [DOI] [PubMed] [Google Scholar]
- 6. Kellum JA, Angus DC. Patients are dying of acute renal failure. Crit Care Med 2002;30:2156–7. 10.1097/00003246-200209000-00041 [DOI] [PubMed] [Google Scholar]
- 7. Srisawat N, Kellum JA. Acute kidney injury: definition, epidemiology, and outcome. Curr Opin Crit Care 2011;17:548–55. 10.1097/MCC.0b013e32834cd349 [DOI] [PubMed] [Google Scholar]
- 8. Depner TA. "Artificial" hemodialysis versus "natural" hemofiltration. Am J Kidney Dis 2008;52:403–6. 10.1053/j.ajkd.2008.07.007 [DOI] [PubMed] [Google Scholar]
- 9. Bellomo R, Tipping P, Boyce N. Continuous veno-venous hemofiltration with Dialysis removes cytokines from the circulation of septic patients. Crit Care Med 1993;21:522–6. 10.1097/00003246-199304000-00011 [DOI] [PubMed] [Google Scholar]
- 10. Heering P, Morgera S, Schmitz FJ, et al. . Cytokine removal and cardiovascular hemodynamics in septic patients with continuous venovenous hemofiltration. Intensive Care Med 1997;23:288–96. 10.1007/s001340050330 [DOI] [PubMed] [Google Scholar]
- 11. Hoffmann JN, Hartl WH, Deppisch R, et al. . Effect of hemofiltration on hemodynamics and systemic concentrations of anaphylatoxins and cytokines in human sepsis. Intensive Care Med 1996;22:1360–7. 10.1007/BF01709552 [DOI] [PubMed] [Google Scholar]
- 12. Honore PM, Joannes-Boyau O. High volume hemofiltration (HVHF) in Sepsis: a comprehensive review of rationale, clinical applicability, potential indications and recommendations for future research. Int J Artif Organs 2004;27:1077–82. [DOI] [PubMed] [Google Scholar]
- 13. Rimmelé T, Kellum JA. High-volume hemofiltration in the intensive care unit: a blood purification therapy. Anesthesiology 2012;116:1377–87. 10.1097/ALN.0b013e318256f0c0 [DOI] [PubMed] [Google Scholar]
- 14. Ronco C, Bellomo R, Homel P, et al. . Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet 2000;356:26–30. 10.1016/S0140-6736(00)02430-2 [DOI] [PubMed] [Google Scholar]
- 15. Saudan P, Niederberger M, De Seigneux S, et al. . Adding a Dialysis dose to continuous hemofiltration increases survival in patients with acute renal failure. Kidney Int 2006;70:1312–7. 10.1038/sj.ki.5001705 [DOI] [PubMed] [Google Scholar]
- 16. Bouman CS, Oudemans-Van Straaten HM, Tijssen JG, et al. . Effects of early high-volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: a prospective, randomized trial. Crit Care Med 2002;30:2205–11. 10.1097/00003246-200210000-00005 [DOI] [PubMed] [Google Scholar]
- 17. Bellomo R, Cass A, Cole L, et al. . Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 2009;361:1627–38. 10.1056/NEJMoa0902413 [DOI] [PubMed] [Google Scholar]
- 18. Palevsky PM, Zhang JH, O’Connor TZ, et al. . VA/NIH Acute Renal Failure Trial Network. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 2008;359:7–20. 10.1056/NEJMoa0802639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tolwani AJ, Campbell RC, Stofan BS, et al. . Standard versus high-dose CVVHDF for ICU-related acute renal failure. J Am Soc Nephrol 2008;19:1233–8. 10.1681/ASN.2007111173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Casey ET, Gupta BP, Erwin PJ, et al. . The dose of continuous renal replacement therapy for acute renal failure: a systematic review and meta-analysis. Ren Fail 2010;32:555–61. 10.3109/08860221003728739 [DOI] [PubMed] [Google Scholar]
- 21. Negash DT, Dhingra VK, Copland M, et al. . Intensity of continuous renal replacement therapy in acute kidney injury in the intensive care unit: a systematic review and meta-analysis. Vasc Endovascular Surg 2011;45:504–10. 10.1177/1538574411407935 [DOI] [PubMed] [Google Scholar]
- 22. Van Wert R, Friedrich JO, Scales DC, et al. . High-dose renal replacement therapy for acute kidney injury: systematic review and meta-analysis. Crit Care Med 2010;38:1360–9. 10.1097/CCM.0b013e3181d9d912 [DOI] [PubMed] [Google Scholar]
- 23. Joannes-Boyau O, Honoré PM, Perez P, et al. . High-volume versus standard-volume haemofiltration for septic shock patients with acute kidney injury (IVOIRE study): a multicentre randomized controlled trial. Intensive Care Med 2013;39:1535–46. 10.1007/s00134-013-2967-z [DOI] [PubMed] [Google Scholar]
- 24. Zhang P, Yang Y, Lv R, et al. . Effect of the intensity of continuous renal replacement therapy in patients with Sepsis and acute kidney injury: a single-center randomized clinical trial. Nephrol Dial Transplant 2012;27:967–73. 10.1093/ndt/gfr486 [DOI] [PubMed] [Google Scholar]
- 25. Higgins JPT. Cochrane collaboration handbook for systematic reviews of interventions Version 5.1.0. Cochrane Collab 2011. www.cochrane-handbook.org [Google Scholar]
- 26. Boussekey N, Chiche A, Faure K, et al. . A pilot randomized study comparing high and low volume hemofiltration on vasopressor use in septic shock. Intensive Care Med 2008;34:1646–53. 10.1007/s00134-008-1127-3 [DOI] [PubMed] [Google Scholar]
- 27. Vesconi S, Cruz DN, Fumagalli R, et al. . Delivered dose of renal replacement therapy and mortality in critically ill patients with acute kidney injury. Crit Care 2009;13:R57 10.1186/cc7784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sterne JA, Sutton AJ, Ioannidis JP, et al. . Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 29. Zhang L, Yang J, Eastwood GM, et al. . Extended daily dialysis versus continuous renal replacement therapy for acute kidney Injury: a meta-analysis. Am J Kidney Dis 2015;66:322–30. 10.1053/j.ajkd.2015.02.328 [DOI] [PubMed] [Google Scholar]
- 30. Friedrich JO, Wald R, Bagshaw SM, et al. . Hemofiltration compared to hemodialysis for acute kidney injury: systematic review and meta-analysis. Crit Care 2012;16:R146 10.1186/cc11458 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-014171supp001.pdf (213.2KB, pdf)