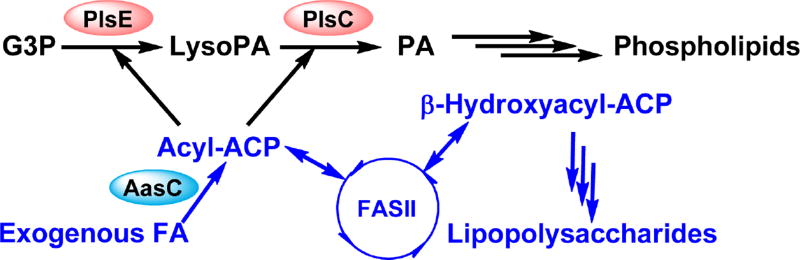
Fig. 3. Phosphatidic acid synthesis and exogenous fatty acid incorporation in C. trachomatis.
Phosphatidic acid is synthesized from the successive acylation of G3P by the G3P acyltransferase PlsE and 1-acyl-sn-glycerol-3-phosphate acyltransferase PlsC in C. trachomatis. The C. trachomatis PlsE is a soluble G3P acyltransferase. Host fatty acids are converted into acyl-ACP by the C. trachomatis acyl-ACP synthetase (AasC). The C. trachomatis acyl-ACP synthetase and lysophospholipid acyltransferase are encoded as separate enzymes rather than a bifunctional enzyme as found in E. coli. This organizational change allows the resulting acyl-ACP to undergo similar fates as endogenously synthesized acyl-ACP: elongated by the FASII or used as an acyl-donor by the acyltransferases. Despite actively incorporating host fatty acids for phospholipid synthesis, C. trachomatis cannot bypass FabI inhibition. The 2-position of C. trachomatis phospholipids has high preference for anteiso15:0 fatty acid, which cannot be obtained from the host.