Fig. 7.
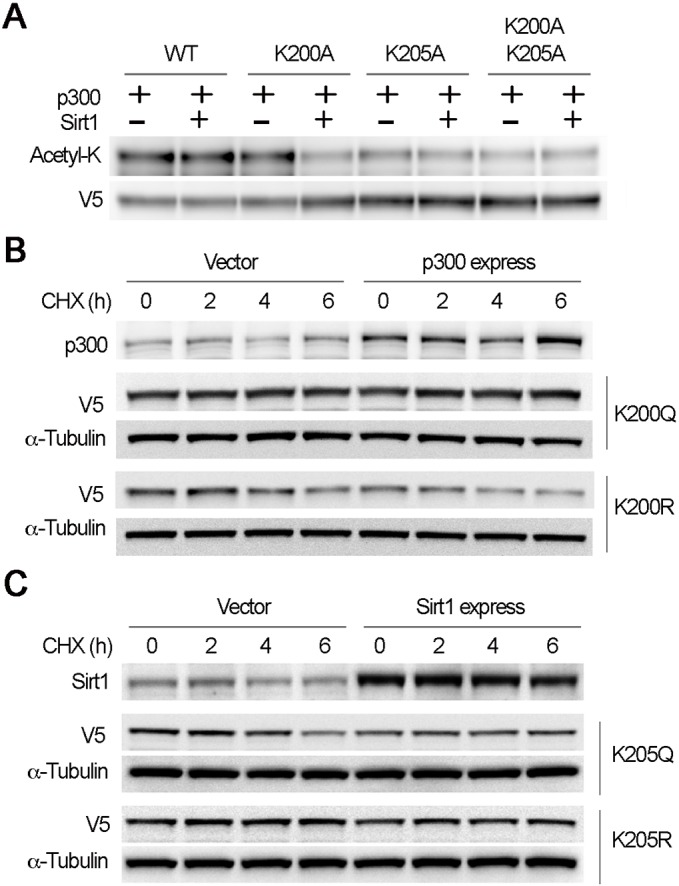
Sequential Sirt1-mediated deacetylation on K200 and p300-mediated acetylation on K205 prepare the IKxxxIK motif for Fbxl17 binding. (A) TnT-synthesized proteins (WT, K200A, K205A and K200A/K205A mutant PRMT1) were preacetylated with recombinant p300 for 1 h at 25°C. The WT and mutant PRMT1 proteins were pulled down by V5 antibody. The pulled-down products were subjected to Sirt1-mediated deacetylation for 1 h at 25°C. The proteins were immunoblotted with acetyl-K and V5 antibodies. (B) Acetylation mimic (K200Q) or deacetylation mimic (K200R) of mutant PRMT1 were co-transfected with empty vector (right panels) or p300 expression constructs (left panels) as indicated. After 24 h the cells were treated with CHX for different time phases. Cell lysates were analyzed by immunoblotting with V5, p300 and α-tubulin antibodies. (C) The half-life of acetylation mimic (K205Q) and deacetylation mimic (K205R) of mutant PRMT1 were co-transfected with empty vector (right panels) or Sirt1 expression constructs (left panels) as indicated. After 24 h, the cells were treated with CHX for various time points. Cell lysates were analyzed by immunoblotting with V5, Sirt1 and α-tubulin antibodies. The results are representative of n=3 experiments.
