Abstract
Background
Delineating specific clinical phenotypes of anxiety disorders is a crucial step toward better classification and understanding of these conditions. The present study sought to identify differential aversive responses to predictable and unpredictable threat of shock in healthy comparisons and in non-medicated anxiety patients with and without a history of panic attacks (PAs).
Method
143 adults (72 healthy controls; 71 patients with generalized anxiety disorder (GAD) or/and social anxiety disorder (SAD), 24 with and 47 without PAs) were exposed to three conditions: 1) predictable shocks signaled by a cue, 2) unpredictable shocks, and 3) no shock. Startle magnitude was used to assess aversive responses.
Results
Across disorders, a PA history was specifically associated with hypersensitivity to unpredictable threat. By disorder, SAD was associated with hypersensitivity to predictable threat, whereas GAD was associated with exaggerated baseline startle.
Conclusions
These results identified three physiological patterns. The first is hypersensitivity to unpredictable threat in individuals with PAs. The second is hypersensitivity to predictable threat, which characterizes SAD. The third is enhanced baseline startle in GAD, which may reflect propensity for self-generated anxious thoughts in the absence of imminent danger. These results inform current thinking by linking specific clinical features to particular physiology profiles.
Keywords: Anxiety disorder, panic attack, anxiety, fear, predictability, startle
Introduction
Research on pathophysiology and biomarkers informs the search for new treatments for anxiety disorders (1-3). Pathological anxiety can be conceptualized as excessive fear or anxiety in response to threats (4), which manifest in various ways at both the behavioral and neural level (5). Therefore, the physiological correlates of fear and anxiety may be particularly useful biomarkers. The present study compares physiologic responding to predictable and unpredictable threat in individuals with generalized anxiety disorder (GAD), social anxiety disorder (SAD), panic attacks (PAs), or no mental disorder.
The startle reflex indexes heterogeneous features of aversive states as they manifest across species. Particularly consistent results arise in research on predictable and unpredictable threats, which evoke aversive responses with overlapping but distinct neural origins. Specifically, while an imminent and predictable threat induces a phasic fear response mediated by the amygdala, unpredictable threat induces a more sustained anxiety state mediated by the bed nucleus of the stria terminalis (BNST) (5). This distinction between fear, a response to “acute threat”, and anxiety, a response to “potential harm”, is reflected in the Research Domain Criteria (RDoC) (3).
In a translational extension of this approach, we developed a protocol to examine responses to predictable and unpredictable shock in humans (6, 7). In the so-called NPU threat test (N, P, and U standing for Neutral, Predictable, and Unpredictable) paradigm, fear and anxiety are operationally defined as the increase in startle magnitude from a neutral condition to a period of predictable (i.e., fear-potentiated startle) and unpredictable (i.e., anxiety-potentiated startle) shock anticipation, respectively.
In separate studies, we used the NPU test to examine physiologic responses in various clinical conditions. We reported a selective hypersensitivity to unpredictable but not predictable threat in panic disorder (PD) and posttraumatic stress disorder (PTSD) (8, 9) and normal responses to predictable and unpredictable threats in generalized anxiety disorders (GAD) (9). This latter result was contrary to the expectation that GAD would be associated with exaggerated response to unpredictable threat, given that core GAD symptoms include sustained anticipatory anxiety and uncontrollable worry (10-12).
A potential explanation for these negative results in GAD is that we used mild aversive stimuli (i.e., airblasts directed to the neck, loud unpleasant sounds) rather than shocks, which are more unpleasant and evoke robust anxiety-potentiated startle (6). To investigate this possibility, the current study used shock. It was expected that individuals with GAD would be hypersensitive to unpredictable shocks.
Little is currently known about aversive responses during shock anticipation in SAD. Individuals with SAD show exaggerated startle potentiation to social threat (13, 14) but not to commonly shared threat (e.g., physical attack by animal or human) (13). However, to the best of our knowledge, no study has yet been published on startle reactivity during shock anticipation in SAD. Neuroimaging studies point to hyperactivity, especially in the amygdala, in response to discrete (i.e., predictable) emotional stimuli, including non-social stimuli, in SAD (15, 16). Based on these observations, we hypothesized that SAD would be associated with a hypersensitivity to predictable threat.
Panic attacks (PAs) consist of abrupt, overwhelming fear and terror. Although PAs are the hallmark of PD, they are also among the most common symptoms in anxiety disorders and other psychiatric disorders (17). In fact, PAs constitute a nonspecific risk factor for psychopathology (17), which has led to their inclusion as a specifier in DSM-5 (4). Therefore, a better understanding of PAs could have far-reaching implications for our comprehension of psychiatric conditions.
As aforementioned, we have reported hypersensitivity to unpredictable threat in PD (8). Similarly, another group, also relying on the NPU threat test, has reported that hypersensitivity to unpredictable threat, but not predictable threat, was associated with increased familial liability for PD (18). Given that PAs define PD, these results raise the possibility of an association between PAs and unpredictable threat rather than predictable threat (5, 19). In fact, we have obtained preliminary evidence for such an association in an ongoing family study of mood and anxiety disorders (20). The present study, therefore, tested the hypothesis that PAs are associated with hypersensitivity to unpredictable threat by comparing individuals with GAD and/or SAD with and without a history of PAs.
To summarize, the present work sought to identify potential clinical phenotypes by examining the pattern of responses to predictable and unpredictable threats in individuals with GAD and/or SAD, with or without a history of PAs. We hypothesized that compared to controls, a history of PAs or a diagnosis of GAD would be associated with enhanced anxiety-potentiated startle to unpredictable threat, but with normal fear-potentiated startle to predictable threat. Finally, we hypothesized that SAD would be associated with normal anxiety-potentiated startle, but enhanced fear-potentiated startle, reflecting a hypersensitivity to predictable threat.
Methods and Materials
Participants
71 medication-free patients with an anxiety disorder (51 women) and 74 healthy controls (51 women) participated in the study. Participants were recruited from the Washington DC metropolitan area (USA) through flyers, email lists, and newspaper advertisements. There were three lines of recruitment, 1) for “anxiety and worry problems” aimed at recruiting individuals with an anxiety disorder, 2) individuals who had experienced panic attacks, and 3) for healthy controls. Following an initial telephone screen, participants visited the NIH for comprehensive screening by a psychologist and a physician or a nurse practitioner. The patients had a diagnosis of GAD (N=27), SAD (N=21), or had comorbid GAD and SAD (N=24). All patients with SAD (except one in the GAD/ SAD group) had generalized social anxiety disorder. No other current Axis I psychiatric disorder, or past psychosis as assessed by the Structural Clinical Interview for DSM-IV Axis I disorders (SCID) (21) were allowed. 45 patients had never been medicated with anxiolytics and of the 26 who had taken anxiolytics, only 8 took medication in the past 1-6 months. All patients were free of medication for at least 3 weeks prior to testing. Healthy comparisons had no current or past Axis I psychiatric diagnosis according to the SCID. A subsample of the anxious patients (N=24) reported having experienced unexpected PAs (18 reported at least 2 and 6 reported at least 1) (Table 1). PAs were symptoms of intense fear as described in DSM IV. Two comparisons subjects reported at least 2 PAs. However, due to their small numbers, the two comparisons with PAs were excluded, though analyses including or excluding these subjects generated similar conclusions, as presented in Supplemental Information (Table S1). Mean age (Table 1) did not significantly differ across groups (t[142]=.40, ns). All subjects were free of illicit substances, as per urine screen. None of the subjects had participated in a NPU threat test before. Written informed consent was obtained after detailed description of the study.
Table 1.
Demographic information: Mean and (sem) age (years), state anxiety, and trait anxiety
| Controls | GAD | SAD | GAD/SAD | ||||
|---|---|---|---|---|---|---|---|
| Panic attacks | No | No | Yes | No | Yes | No | Yes |
| N | 72 | 16 | 11 | 15 | 6 | 17 | 7 |
| Age | 35.0 (13.0) | 30.5 (2.4) | 35.0 (3.8) | 29.2 (2.8) | 31.3 (2.7) | 29.0 (2.1) | 29.7 (3.4) |
| Sate anxietya | 25.0 (.7) | 44.1 (3.0) | 37.8 (3.8) | 39.2 (2.8) | 42.5 (3.3) | 49.5 (2.7) | 45.8 (3.8) |
| Trait anxietyb | 39.4 (.4) | 47.6 (2.6) | 45.0 (3.8) | 46.0 3.2) | 52.0 (3.9) | 55.1 (2.5) | 56.7 (3.2) |
| BAIc | 2.2 (.9) | 12.3 (3.1) | 13.0 (3.7) | 11.1 (2.1) | 9.8 (2.0) | 20.1 (2.2) | 15.0 (.4) |
| BDId | .4 (.2) | 6.4 (1.4) | 7.5 (1.8) | 6.4 (1.4) | 4.8 (2.4) | 11.6 (1.6) | 14.1 (3.2) |
| PSWQe | 32.8 (2.5) | 53.4 (5.1) | 58.1 (5.4) | 53.4 (5.1) | 63.0 (5.8) | 65.5 (1.7) | 65.1 (4.3) |
GAD, generalized anxiety disorder; SAD, social anxiety disorder.
Spielberger state portion of the State-Trait Anxiety Inventory (22)
Spielberger trait portion of the State-Trait Anxiety Inventory (22)
Beck Anxiety Inventory (24)
Beck Depression Inventory (23)
Penn State Worry Questionnaire (25).
Procedure
On the day of the physical and psychiatric screen, participants filled out the trait portion of the State-Trait Anxiety Inventory (STAI-trait) (22). Prior to the NPU threat test, participants completed the state portion of the STAI (STAI-state), the Beck Depression Inventory (BDI) (23), the Beck Anxiety Inventory (BAI) (24), the Penn State Worry Questionnaire (PSWQ) (25), and then were fitted with two electrodes under their left eye to record the electromyographic eyeblink/startle reflex. To assess baseline startle, participants were exposed to nine acoustic startle stimuli every 18–23 sec via headphones (habituation startle). A shock work-up was also implemented to set the intensity of shock to a mildly painful level.
The NPU threat procedure is shown in Fig S1 (Supplemental Information). It is described in details in (7) and was similar to that used in our previous clinical and psychopharmacological studies (9, 26). Participants were given explicit instructions regarding the threat test, which consisted of three 150-sec conditions: 1) no threat (N); 2) predictable threat (P); and 3) unpredictable threat (U). In each 150-sec condition, an 8-sec duration cue was presented four times. The cues differed in color and shape for each condition (e.g., blue square for N, red circle for P, green triangle for U). The cues signaled the possibility of receiving a shock in the P condition only, but carried no information in the N and U conditions. During the experiment, instructions were continuously displayed showing: “no shock” (N), “shock during shape” (P), or “shock at any time” (U). In each condition, six acoustic startle stimuli were delivered, three during inter-trial intervals (ITI, i.e., cue-free periods) and one during three of the four cues, 5-7 sec following cue onset.
There were two runs separated by a 5 min break. Each run started with the delivery of four startle stimuli (pre-test startle) and consisted of three N, two P, and two U conditions in one of the following two orders: P N U N U N P or U N P N P N U. Each participant received both orders, with one-half of them starting with P and the other one-half starting with U. One shock was administered in each individual P and U condition for a total of four shocks in P and four shocks in U. In each P, the shock was randomly associated with one of the four threat cues, being administered 7.5 s after the onset, i.e., 500 ms before the termination, of that cue. In each U, the shock was given either 7 or 10 s after the termination of a cue. No startle stimulus was delivered within 8 sec after a shock to avoid potential short-term startle sensitization.
After each run, subjects retrospectively rated their anxiety level in the presence and absence of the cue in each condition (N, P, U) on a Likert scale ranging from 0 (not at all fearful/anxious) to 10 (extremely fearful/anxious).
Stimuli and Physiological Responses
Stimulation and recording were controlled by a commercial system (Contact Precision Instruments, London, England). The acoustic startle stimulus was a 40-ms, 103-dB burst of white noise. The eyeblink reflex was recorded with electrodes placed under the left eye. Amplifier bandwidth was set to 30-500 Hz.
Data Analysis
Peak blink amplitude was determined in the 20-100-ms time frame following stimulus onset relative to a 50-ms baseline EMG. The analysis of baseline eyeblink/startle magnitude was conducted using the raw score (μV). Subsequently, and because of group differences in baseline startle reactivity (See Results), eyeblink magnitudes were standardized using within-subjects T-scores ([Z scores × 10] + 50). Startle magnitudes and subjective ratings were averaged across conditions, separately for cues and ITI.
As in our previous studies (e.g., (9, 26)) and consistent with our a priori hypotheses, we examined fear and anxiety separately. Fear-potentiated startle was operationally defined as the increased startle magnitude from ITI to the threat cue in P and anxiety-potentiated startle was operationally defined as the increased ITI startle magnitude from N to U. The data were analyzed with analyses of variance with repeated measure (rANOVAs) and t-tests. For each variable, two analyses were conducted. The first contrasted the healthy comparison group to the DSM-5 disorders (i.e., control, GAD, SAD, GAD/SAD) and the second compared patients with and without PAs across the entire patients groups. Alpha was set at .05 for all statistical tests.
Results
Demographics
Demographic data are presented in Table 1. The data were analyzed with a one-way ANOVA with group (control, GAD, SAD, GAD/SAD) as a factor. As expected patients had higher state anxiety, trait anxiety, BAI, BDI, and PSWQ scores compared to controls (all p>.02). Among the anxious groups the comorbid GAD/SAD group had higher state anxiety, trait anxiety, BAI, BDI, and PSWQ scores compared to the SAD group (all p<.05) and higher state and BDI scores compared to the GAD group (all p<.05). Within the entire patient group, there was not significant difference among patients with and without PAs (all p>.1).
Startle
Startle reactivity expressed in μV and T-scores in all groups and conditions are presented in Supplemental information (Tables S1 and S2).
Baseline startle
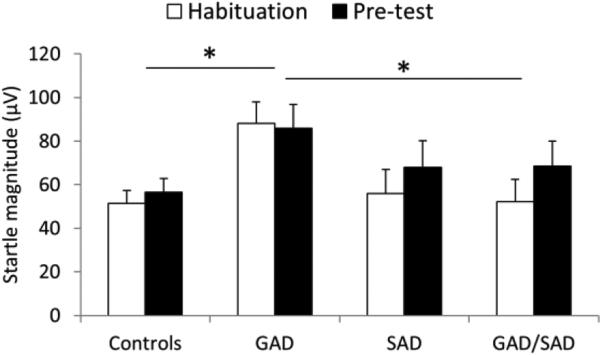
To compare startle reactivity among groups, the raw startle magnitudes were averaged over the 9 initial startle responses (habituation startle) and over the 4 first startle trials (pre-test startle) of the 1st threat run (Fig. 1). Startle scores were analyzed with a group (control, GAD, SAD, GAD/SAD) × time (habituation, pre-test) rANOVA. There were main effects of group (F[3,140]=2.8, p=.04) and time (F[1,140]=6.0, p=.01), with no group × time interaction (F[3,140]=1.4, ns). Post-hoc Tukey's Studentized Range (HSD) tests showed that the Group main effect reflected larger startle in the GAD relative to healthy comparison (p=.008) and GAD/SAD groups (p=.02). The GAD vs. SAD comparison was a trend (p=.068). The time main effect reflected increased startle magnitude from habituation to pre-test, probably caused by an increased in threat proximity. The group main effect remained significant (F[3,138]=3.2, p=.02) when age and sex were used as covariates.
Fig. 1.
Baseline startle magnitude in the anxious patients and in the controls during startle habituation and during pre-test. Error bars are standard error of the mean (sem). * for p<.05. Overall startle magnitude was significantly larger in the GAD group compared to the Control and to the GAD/SAD groups. GAD, generalized anxiety disorder; SAD, social anxiety disorder.
Analysis of the effect of PAs in the anxious patients was conducted using a PA (yes, no) × time (habituation, pre-test) ANOVA. Results showed no effect of PAs on baseline startle (PA main effect, F[1,70]=2.7, ns).
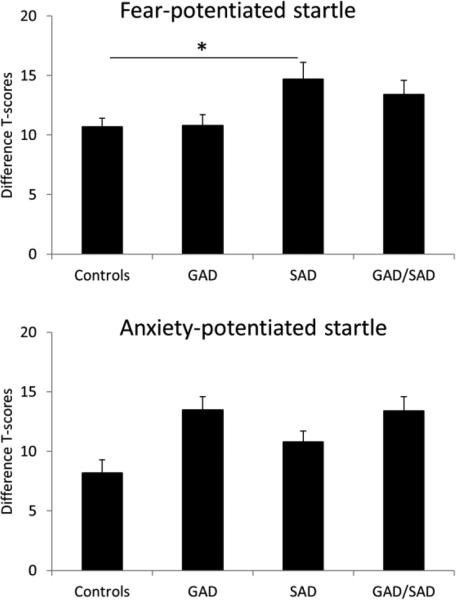
Fear-potentiated startle
Fear-potentiated startle (difference T-scores startle during cue minus startle during ITI in the predictable condition: Fig. 2, top) was analyzed with a one-way group (control, GAD, SAD, GAD/SAD) ANOVA. The group main effect was significant (F[3,140]=3.3, p=.022), reflecting larger fear-potentiated startle in the SAD group compared to controls (p=.034), with no other group difference. These effects remained significant (main group effect: F[3,138]=3.0, p=.033; control vs. SAD: p=.055) when age and sex where used as covariates.
Fig. 2.
Fear-potentiated startle (top) and anxiety-potentiated startle (bottom) in the anxious patients and in the controls. Error bars are sem. * for p<.05. Fear-potentiated startle was significantly larger in the SAD group compared to the Control group.
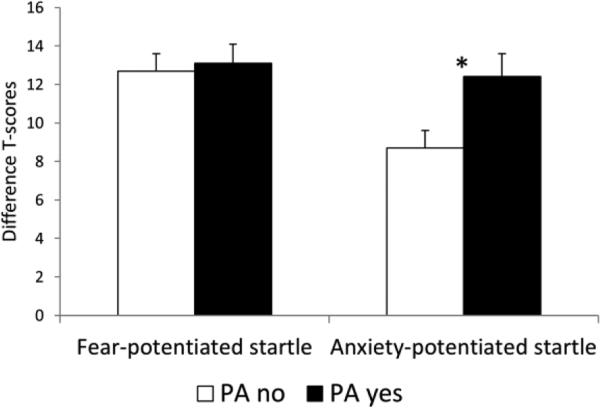
Fear-potentiated startle in the patients with and without a history of PAs was analyzed with a t-test. Results showed no effect of PAs on fear-potentiated startle (t[70]=.25, ns) (Fig. 3, top).
Fig. 3.
Fear-potentiated startle and anxiety-potentiated startle in the anxious patients separated according to the presence or absence of PAs. Error bars are sem. * for p<.05. Anxiety-potentiated startle was significantly larger in the patients with a history of PAs compared to the patients without a history of PAs.
Anxiety-potentiated startle
Anxiety-potentiated startle (difference T-score in ITI startle during the unpredictable condition minus the neutral condition: Fig. 2, bottom) analyzed with a one-way group (control, GAD, SAD, GAD/SAD) ANOVA. Results showed a significant main group effect (F[3,140]=2.9, p=.038), due to a trend for larger anxiety-potentiated startle in the GAD group compared to controls (p=.058), with no other group difference. The Group main effect remained significant (F[3,138]=2.9, p=.036) when age and sex where used as covariates.
Anxiety-potentiated startle in the patients with and without a history of PAs was analyzed with a t-test. As predicted, results showed larger anxiety-potentiated startle in patients with PAs vs. without PAs (t[70]=2.5, p=01) (Fig. 3, bottom). The PA main effect remained significant (F[1,65]=5.3, p=.02) when age, sex, trait anxiety, and BDI were used as covariates.
Subjective ratings
Analyses of the retrospective subjective fear and anxiety ratings are shown in Supplemental information. Overall the results show increased subjective fear and anxiety rating in the patients compared to the controls, with no difference between the patients with and without PAs (Table S3).
Discussion
Threat reactivity is a broad construct encompassing responses that could vary with the nature of the threat (3, 5). To identify clinical phenotypes, the present study examined patterns of responses to predictable and unpredictable threats among individuals with no anxiety or various clinical features, including GAD and SAD, with or without or PAs. Distinct patterns manifested in specific clinical states. Individuals with SAD were hypersensitive to predictable but not unpredictable threat, while individuals with GAD showed elevated baseline startle. Responses in the comorbid GAD/SAD group fell between these two groups but did not differ significantly from the control group. Across anxiety disorders, PAs were associated with hypersensitivity to unpredictable but not predictable threat, consistent with other preliminary findings (20).
GAD was associated with heightened baseline startle reactivity, regardless of PAs. It is well established that startle is potentiated in threatening contexts (e.g., associated with shock), even when there is no imminent risk because the threat is remote in time (27-29). In addition, we (9) and others (30) have reported no elevated baseline startle in GAD in innocuous environments. Finally, individuals with GAD are overly sensitive to the perception of threat (31). Thus, elevated baseline startle in the GAD group probably reflects exaggerated anxious apprehensive response to the threatening context.
This hypersensitivity to contextual threat in GAD is a key finding that capture an essential characteristic of GAD, i.e., the propensity to experience negative thoughts and worries about the future (12). We have previously reported similar exaggerated response to contextual threat in major depression, a condition also characterized by excessive negative self-generated thoughts (i.e., rumination) (12, 32), and which may share a common underlying core dysfunction with GAD (33). Future studies should examine whether structures implicated in negative self-generated thoughts, including the medial prefrontal cortex and the posterior cingulate cortex (12), are overly engaged during unpredictable threat in GAD.
Individuals with SAD were hypersensitive to predictable threat but not unpredictable threat. This is a notable finding because we have never found exaggerated response to predictable threat in anxiety disorders, including GAD, PD, and PTSD (8, 9, 28, 29, 34, 35), suggesting that this excessive response may characterize SAD. Neuroimaging studies consistently find exaggerated amygdala response to discrete social and non-social emotional stimuli, suggesting that this structure is implicated in the pathophysiology of SAD (15, 16).
A key finding was the association between PAs and hypersensitivity to unpredictable threat in the anxious subjects. We previously showed a similar hypersensitivity to unpredictable threat not only in PD, but also in PTSD (9), a condition with a high incidence of PAs (36). The identification of a link between PAs and hypersensitivity to unpredictable threat provides new leads to improve our understanding of underlying psychological and neural processes connecting PAs to a variety of clinical problems and risks (4, 17). Arguably, this hypersensitivity may merely reflect greater symptom severity. This is unlikely. A symptom severity interpretation of the results would predict larger anxiety-potentiated startle in anxiety disorders with PAs compared to those without PAs, but also larger anxiety-potentiated startle in anxiety disorders without PAs compared to controls, which was not the case. In addition, the results were unchanged when anxiety (trait anxiety) and depression (BDI) symptoms were taken into account in the statistical analysis.
A crucial question is whether PAs represent a pre-existing vulnerability or a consequence of hypersensitivity to unpredictable threat? Extant theory and data suggest that PAs could cause hypersensitivity to unpredictable threat. Accordingly, the experience of PAs leads to chronic hypervigilance and anticipatory anxiety in an attempt to detect potential interoceptive signs of upcoming unpredictable PAs (37). This, in turn, could sensitize the underlying neural structures mediating response to unpredictable threat.
Alternatively, hypersensitivity to unpredictable threat, due to familial predisposition (18) or environmental factors (e.g., trauma), could trigger an underlying biological diathesis that lowers the threshold for PAs, subsequently increasing their risk of occurrence. Research shows that stressful life events increase the risk of PAs (38, 39) and that, when exposed to a threat context, children of parents with panic disorder exhibit lower anxiety thresholds, as assessed by startle magnitude, compared to children of healthy parents (40). It is therefore possible that, in individuals who are hypersensitive to unpredictable threat, accumulation of mild unpredictable adverse events lowers the threshold for strong arousal reaction (e.g., more readily engages cardio-vascular responses), which increases their sensation of interoceptive symptoms and ultimately leads to PAs.
There is currently not clear neural abnormalities associated with PAs. A recent review of the neuroimaging literature pointed to the amygdala, anterior cingulate cortex and insula (41). Another structure to be considered based on the present result of an association between PAs and hypersensitivity to unpredictable threat is the BNST, given the role of this structure in the response to unpredictable threat in humans and animals (5). Involvement of the BNST is consistent with basic findings in rodents with panicogenic agents, such as CO2. For example, the BNST contains acid-sensing ion channels (ASIC) that can detect CO2-induced acidosis and BNST lesions reduce CO2-evoked fear in animal models (42).
Results must be considered in light of the study's strengths and limitations. A major strength is our reliance on a well-established translational procedure that provides clues as to brain mechanisms engaged by our threat procedure in rodents (5) and in humans (43-45). A second strength is that the NPU threat test has been extensively used by us and others (46-53), providing us with a priori hypotheses. Finally, the anxious patients were off psychoactive medication for at least 1 month prior to testing. A major limitation was the relative insufficient statistical power to reliably examine any of the interaction of Diagnosis × PA. For example, the increased response to unpredictable threat associated with PAs seems to be mostly driven by individuals with GAD or GAD/SAD, as the SAD-only group showed only weak effect of PA on anxiety-potentiated startle (Table 2s). Another limitation was the unequal number of males compared to females in the different groups. However, all the results were confirmed when sex was used as a covariate in the analyses. A third limitation concerns the subjective reports did not match the startle data. However, we and others have frequently observed dissociation between objective and subjective measures in clinical and psychopharmacology studies (26, 54-56). Startle is an online probe of affective reactivity, whereas the subjective reports are retrospective and are subject to interference from recollection. Nonetheless and consistent with published results (8, 9), ratings of anxiety were overall elevated in patients compared to controls with no additional group difference among threat conditions.
To conclude, discovery of clinical biological phenotypes is a key step in improving clinical classification and identifying pathophysiological mechanisms that could be targeted for treatment development. This study shows that hypersensitivity to unpredictable threat may be a clinical phenotype of PAs that cut across anxiety disorders. Possibly, it may also cut across other psychiatric disorders with high incidence of PAs, including PTSD and substance abuses. GAD is associated with hypersensitivity to the threatening context, a vulnerability that may be associated with a tendency for self-generated thoughts (12). Finally, SAD seems uniquely associated with exaggerated fear-potentiated startle in predictable threat. Substantial progress has been made to uncover the underlying neural mechanisms of these different types of threat, linking response to unpredictable threat to the BNST (43) and responses to predictable threat to the amygdala (43). Thus, these results provide clear and testable hypotheses for future investigations.
Supplementary Material
Acknowledgement
This research was supported by the Intramural Research Program of the National Institute of Mental Health [grant number MH002798] (Protocol 03-M-0093; NCT00055224).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE/CONFLICT OF INTEREST
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Kessler RC. The global burden of anxiety and mood disorders: putting the European Study of the Epidemiology of Mental Disorders (ESEMeD) findings into perspective. J Clin Psychiatry. 2007;68(Suppl. 2):10–19. [PMC free article] [PubMed] [Google Scholar]
- 2.Griebel G, Holmes A. 50 years of hurdles and hope in anxiolytic drug discovery. Nat Rev Drug Discov. 2013;12:667–687. doi: 10.1038/nrd4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Medicine. 2013;11:1–8. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Association AP. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. American Psychiatric Association; Washingron, DC: 2013. [Google Scholar]
- 5.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs. anxiety. Neuropsychopharmacol. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behav Neurosci. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- 7.Schmitz A, Grillon C. Assessing fear and anxiety in humans using threat of predictable and unpredictable aversive events (the NPU-threat test). Nature Protoc. 2012;7:527–532. doi: 10.1038/nprot.2012.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. Am J Psychiatry. 2008;165:898–904. doi: 10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biol Psychiatry. 2009;66:47–53. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roemer L, Orsillo SM, Barlow DH. Generalized anxiety disorder. In: Barlow DH, editor. Anxiety and its disorders: the nature and treatment of anxiety and panic. The Guilford Press; New York: 2002. pp. 477–515. [Google Scholar]
- 11.Borkovec TD, Shadick RN, Hopkins M. The nature of normal and pathological worry. In: Rappe RM, Barlow DH, editors. Chronic anxiety: Generalized anxiety disorder and mixed anxiety-depression. Guilford Press; New York: 1991. pp. 29–51. [Google Scholar]
- 12.Perkins AM, Arnone D, Smallwood J, Mobbs D. Thinking too much: self-generated thought as the engine of neuroticism. Trends in Cognitive Sciences. 2015;19:492–498. doi: 10.1016/j.tics.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Lang PJ, McTeague LM. The anxiety disorder spectrum: fear imagery, physiological reactivity, and differential diagnosis. Anxiety Stress Coping. 2009;22:5–25. doi: 10.1080/10615800802478247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lissek S, Levenson J, Biggs A, Johnson LL, Ameli R, Pine DS, et al. Social anxiety disorder is associated with elevated fear-conditioning to socially relevant unconditioned stimuli. Am J Psychiatry. 2008;165:124–132. doi: 10.1176/appi.ajp.2007.06091513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Etkin A, Wager TD. Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freitas-Ferrari MC, Hallak JEC, Trzesniak C, Filho AS, Machado-de-Sousa JP, Chagas MHN, et al. Neuroimaging in social anxiety disorder: A systematic review of the literature. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34:565–580. doi: 10.1016/j.pnpbp.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 17.Craske MG, Kircanski K, Epstein A, Wittchen H-U, Pine DS, Lewis-Fernández R, et al. Panic disorder: a review of DSM-IV panic disorder and proposals for DSM-V. Depression and Anxiety. 2010;27:93–112. doi: 10.1002/da.20654. [DOI] [PubMed] [Google Scholar]
- 18.Nelson BD, McGowan SK, Sarapas C, Robison-Andrew EJ, Altman SE, Campbell ML, et al. Biomarkers of threat and reward sensitivity demonstrate unique associations with risk for psychopathology. J Abn Psychol. 2013;122:662–671. doi: 10.1037/a0033982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revised. Am J Psychiatry. 2000;157:493–505. doi: 10.1176/appi.ajp.157.4.493. [DOI] [PubMed] [Google Scholar]
- 20.Schmitz A, Grillon C, Merikangas KR. Anxiety-potentiated startle as potential endophenotype for anxiety disorders. Biol Psychiatry. 2013;73:51S. [Google Scholar]
- 21.First MB, Spitzer RI, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-V (SCID) American Psychiatric Association; Washington, DC.: 1995. [Google Scholar]
- 22.Spielberger CD. Manual for the State-Trait Anxiety Inventory. Consulting Psychologist Press; Palo Alto, CA: 1983. [Google Scholar]
- 23.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 24.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 25.Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- 26.Grillon C, Hale E, Lieberman L, Davis A, Pine DS, Ernst M. The CRH1 antagonist GSK561679 increases human fear but not anxiety as assessed by startle. Neuropsychopharmacol. 2015;40:1064–1071. doi: 10.1038/npp.2014.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grillon C, Davis M. Fear-potentiated startle conditioning in humans: explicit and contextual cue conditioning following paired vs. unpaired training. Psychophysiology. 1997;34:451–458. doi: 10.1111/j.1469-8986.1997.tb02389.x. [DOI] [PubMed] [Google Scholar]
- 28.Grillon C, Morgan CA. Fear-potentiated startle conditioning to explicit and contextual cues in Gulf war veterans with posttraumatic stress disorder. J Abn Psychol. 1999;108:134–142. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- 29.Grillon C, Morgan CA, Davis M, Southwick SM. Effects of experimental context and explicit threat cues on acoustic startle in Vietnam veterans with posttraumatic stress disorder. Biol Psychiatry. 1998;44:1027–1036. doi: 10.1016/s0006-3223(98)00034-1. [DOI] [PubMed] [Google Scholar]
- 30.Ray WJ, Molnar C, Aikins D, Yamasaki A, Newman MG, Castonguay L, et al. Startle response in generalized anxiety disorder. Depression and anxiety. 2009;26:147–154. doi: 10.1002/da.20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borkovec TD, Alcaine OM, Behar E. Avoidance theory of worry and generalized anxiety disorder. In: Heimberg RG, Turk CL, Mennin DS, editors. Generalized anxiety disorder: advances in research and practice. Guilford; New York: 2004. pp. 77–108. [Google Scholar]
- 32.Kuehner C, Weber I. Responses to depression in unipolar depressed patients: an investigation of Nolen-Hoeksema's response styles theory. Psychological Medicine. 1999;29:1323–1333. doi: 10.1017/s0033291799001282. [DOI] [PubMed] [Google Scholar]
- 33.Krueger RF. The structure of common mental disorders. Archives of General Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- 34.Morgan CA, III, Grillon C, Southwick SM, Davis M, Charney DS. Fear-potentiated startle in posttraumatic stress disorder. Biological Psychiatry. 1995;38:378–385. doi: 10.1016/0006-3223(94)00321-S. [DOI] [PubMed] [Google Scholar]
- 35.Morgan CA, III., Grillon C, Southwick SM, Charney DS. Exaggerated acoustic startle reflex in Gulf war veterans with posttraumatic stress disorder. American Journal of Psychiatry. 1996;153:64–68. doi: 10.1176/ajp.153.1.64. [DOI] [PubMed] [Google Scholar]
- 36.Marshall-Berenz EC, Vujanovic AA, Zvolensky MJ. Main and interactive effects of a nonclinical panic attack history and distress tolerance in relation to PTSD symptom severity. Journal of Anxiety Disorders. 2011;25:185–191. doi: 10.1016/j.janxdis.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouton ME, Mineka S, Barlow DH. A modern learning theory perspective on the etiology of panic disorder. Psychol Rev. 2001;108:4–32. doi: 10.1037/0033-295x.108.1.4. [DOI] [PubMed] [Google Scholar]
- 38.Klauke B, Deckert J, Reif A, Pauli P, Domschke K. Life events in panic disorder—an update on “candidate stressors”. Depression and Anxiety. 2010;27:716–730. doi: 10.1002/da.20667. [DOI] [PubMed] [Google Scholar]
- 39.Moitra E, Dyck I, Beard C, Bjornsson AS, Sibrava NJ, Weisberg RB, et al. Impact of stressful life events on the course of panic disorder in adults. pdf2 puberty. 2011;134:373–376. doi: 10.1016/j.jad.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merikangas KR, Avenevoli S, Dierker L, Grillon C. Vulnerability factors among children at risk for anxiety disorders. Biological Psychiatry. 1999;46:1523–1535. doi: 10.1016/s0006-3223(99)00172-9. [DOI] [PubMed] [Google Scholar]
- 41.Dresler T, Guhn A, Tupak S, Ehlis A-C, Herrmann M, Fallgatter A, et al. Revise the revised? New dimensions of the neuroanatomical hypothesis of panic disorder. Journal of Neural Transmission. 2013;120:3–29. doi: 10.1007/s00702-012-0811-1. [DOI] [PubMed] [Google Scholar]
- 42.Taugher RJ, Lu Y, Wang Y, Kreple CJ, Ghobbeh A, Fan R, et al. The bed nucleus of the stria terminalis is critical for anxiety-related behavior evoked by CO2 and acidosis. The Journal of Neuroscience. 2014;34:10247–10255. doi: 10.1523/JNEUROSCI.1680-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C. Phasic and sustained fear in humans elicits distinct patterns of brain activity. Neuroimage. 2011;1:389–400. doi: 10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C. Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. J Neuroscience. 2008;28:6211–6219. doi: 10.1523/JNEUROSCI.1246-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hasler G, Fromm S, Alvarez RP, Luckenbaugh DA, Drevets WC, Grillon C. Cerebral blood flow in immediate and sustained anxiety. J Neurosci. 2007;27:6313–6319. doi: 10.1523/JNEUROSCI.5369-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson BD, Bishop JR, Sarapas C, Kittles RA, Shankman SA. Asians demonstrate reduced sensitivity to unpredictable threat: A preliminary startle investigation using genetic ancestry in a multiethnic sample. Emotion. 2014;14:615–623. doi: 10.1037/a0035776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson BD, Hidges A, JHajcak G, Shankman SA. Anxiety sensitivity and the anticipation of predictable and unpredictable threat: Evidence from the startle response and event-related potentials. J Anxiety Disord. 2015;33:62–71. doi: 10.1016/j.janxdis.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chamberlain P, Rodgers J, Crowley M, White S, Freeston M, South M. A potentiated startle study of uncertainty and contextual anxiety in adolescents diagnosed with autism spectrum disorder. Molecular Autism. 2013;4:31. doi: 10.1186/2040-2392-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gorka S, Nelson B, Sarapas C, Campbell M, Lewis G. Relation Between Respiratory Sinus Arrythymia and Startle Response During Predictable and Unpredictable Threat. Journal of Psychophysiology. 2013;27:95–104. doi: 10.1027/0269-8803/a000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nelson BD, Hajcak G, Shankman SA. Event-related potentials to acoustic startle probes during the anticipation of predictable and unpredictable threat. Psychophysiology. 2015 doi: 10.1111/psyp.12418. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 51.Bradford DE, Kaye JT, Curtin JJ. Not just noise: Individual differences in general startle reactivity predict startle response to uncertain and certain threat. Psychophysiology. 2014;51:407–411. doi: 10.1111/psyp.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schroijen M, Fantoni S, Rivera C, Vervliet B, Schruers K, van den Bergh O, et al. Defensive activation to (un)predictable interoceptive threat: The NPU respiratory threat test (NPUr). Psychophysiology. 2016 doi: 10.1111/psyp.12621. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 53.Gorka SM, Nelson BD, Shankman SA. Startle response to unpredictable threat in comorbid panic disorder and alcohol dependence. Drug and Alcohol Dependence. 2013;132:216–222. doi: 10.1016/j.drugalcdep.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harmer CJ, Rogers RD, Tunbridge E, Cowen PJ, Goodwin GM. Tryptophan depletion decreases the recognition of fear in female volunteers. Psychopharmacol. 2003;167:411–417. doi: 10.1007/s00213-003-1401-6. [DOI] [PubMed] [Google Scholar]
- 55.Grillon C, Chavis C, Covington MS, Pine DS. Two-week treatment with citalopram reduces contextual anxiety but not cued fear. Neuropsychopharmacol. 2009;34:964–971. doi: 10.1038/npp.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lieberman LC, Gorka SM, Sarapas C, Shankman SA. Cognitive flexibility mediates the relation between intolerance of uncertainty and safety signal responding in those with panic disorder. Cogn Emot. 2015 doi: 10.1080/02699931.2015.1067189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.