Abstract
BACKGROUND AND PURPOSE
Intra-arterial recanalization postprocedural imaging in stroke patients can result in diagnostic complications due to hyperdensities on noncontrast computed tomography (CT), which may represent either contrast extravasation or intracranial hemorrhage. If these lesions are hemorrhage, then they are risk factors becoming symptomatic, which, if not distinguished, can alter clinical management. We investigate the effects of iodinated contrast on postprocedural magnetic resonance imaging (MRI) and prevalence of equivocal imaging interpretations of postprocedural extravasated contrast versus hemorrhage while identifying protocol pitfalls.
METHODS
We identified 10 patients diagnosed with ischemic stroke who underwent intra-arterial recanalization in a 5-year period. These patients demonstrated a hyperdensity on a postprocedural CT within 24 hours, underwent an MRI within 48 hours, and an additional confirmatory noncontrast CT at least 72 hours postprocedure.
RESULTS
Postprocedural MRI in all 10 stroke patients demonstrated T1 - and T2-relaxation time changes due to residual iodine contrast agents. This lead to false positive postprocedural hemorrhage MRI interpretations in 2/10 patients, 3/10 false negative interpretations of contrast extravasation, and 5/10 equivocal interpretations suggesting extravasation or hemorrhage. Of these five cases, two were performed with gadolinium.
CONCLUSION
MRI done within 48 hours postprocedure can lead to false positive hemorrhage or false negative contrast extravasation interpretations in stroke patients possibly due to effects from the administered angiographic contrast. Additionally, MRI should be done both after 72 hours for confirmation and without gadolinium contrast as the effects of the gadolinium contrast and residual angiographic contrast could lead to misdiagnosis.
Keywords: Stroke, extravasation, hemorrhage, thrombectomy, CT, MRI
Introduction
Intra-arterial recanalization has become a mainstay of stroke therapy.1–6 However, postprocedural imaging has resulted in diagnostic difficulties due to the presence of hyperdense lesions on noncontrast computed tomography (CT).1,9–11,14–17 These hyperdense lesions on postprocedural CT after intra-arterial recanalization by thrombolysis or mechanical thrombectomy are common occurrences.1,9–11,14–17 However, according to prior studies, a visualized hyperdense area on CT images is a risk factor for both hemorrhagic transformation as well as symptomatic postprocedural iatrogenic hemorrhage. For that reason, discerning whether these hyperdense lesions on CT are hemorrhage or solely angiographic contrast extravasation is crucial in stroke or trauma with potentially significant clinical ramifications. Postprocedural iatrogenic hemorrhage has an incidence of at least 10.9%12 with some studies showing up to 15% and, if symptomatic, has a mortality of up to 83%.29 Magnetic resonance imaging (MRI) performed post intra-arterial recanalization, which uses angiographic iodine contrast media during the procedure, could potentially lead to false positive intracranial hemorrhage interpretations or false negative interpretations of contrast extravasation by neuroradiologists.7,8 It is known that iodinated contrast agents can have imaging effects of at least 48 hours.24–27 In this retrospective single center study, we analyzed a cohort of 10 ischemic stroke patients who underwent intra-arterial recanalization with a post-procedural head CT done within 24 hours, followed by MRI within 48 hours and a confirmatory CT without contrast done 72 hours postprocedure. The goal of this study was to investigate the effects of iodinated contrast on postprocedural MRI, understand the prevalence of equivocal interpretation of hyperdense areas, and identify any postprocedural protocol-related pitfalls.
Materials and Methods
Patient Selection
In this single institution Internal Review Board (IRB) approved retrospective study (IRB#14-1544), we performed a database search using iSite Illuminate (Philips Healthcare, Best, The Netherlands) with the following search terms:
thrombectomy, or
thrombolysis, or
embolectomy, or
embolysis.
This database search yielded 500 eligible patients with age range from 30 to 75 years after ischemic stroke from between December 1, 2010 to December 1, 2015. From these 500 patients, a subgroup of patients was selected in which a postprocedural CT was done within 24 hours as well as an MRI was done within 48 hours of the recanalization procedure (Fig 1). This strict selection criteria yielded a 10 patient subgroup with five males and five females. The eventual diagnosis was confirmed on noncontrast CTs done at least 72 hours after the procedure (Fig 2). The confirmation of extravasation or hemorrhage is based on the final impression of the post 72 hour CT report done by experienced neuroradiologists.
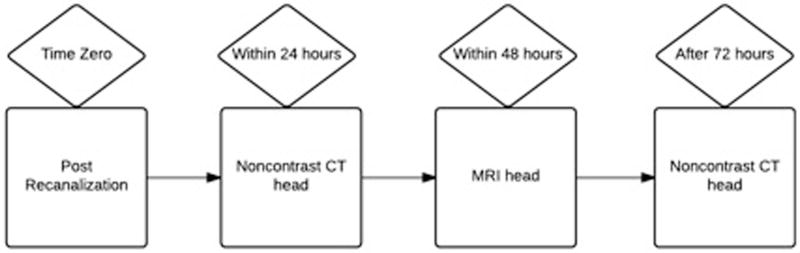
Fig 1.
Inclusion criteria of our study for stroke patients post recanalization to qualify for a retrospective analysis. In order to qualify for this retrospective analysis, a stroke patient must have undergone a noncontrast head CT within 24 hours of recanalization procedure. Additionally, an MR brain without contrast must have been completed within 48 hours of the procedure for further characterization. Finally, another noncontrast head CT must have been done at least after 72 hours for confirmation of hemorrhage or contrast extravasation.
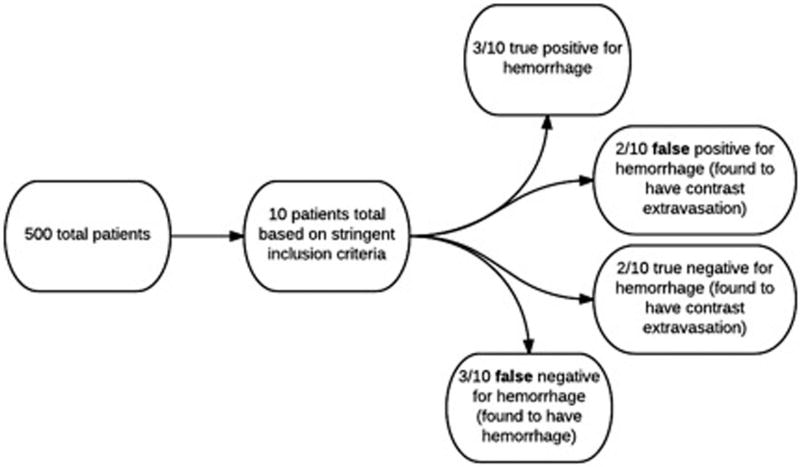
Fig 2.
In total, 500 eligible patients were identified with a database search in this retrospective study. Of those 500 patients, 10 patients qualified based on the stringent imaging inclusion criteria (Fig 1). Three out of 10 patients had a working diagnosis of postprocedural hemorrhage and were deemed true positives. Two out of 10 patients had a working diagnosis of postprocedural hemorrhage but were found to have contrast extravasation and were characterized as false positives. Two of 10 patients had a working diagnosis of contrast extravasation and were stratified as true negatives. Finally, three out of 10 patients had a working diagnosis of contrast extravasation but were found to have hemorrhage. These cases were deemed false negatives.
Analysis
Phantom Study
An MR phantom study with different concentrations of the iodinated contrast agent Omnipaque 350 [Iohexol] (GE Healthcare, Chicago, IL, USA) was performed. Iodinated contrast was diluted with physiological saline solution to create different concentrations ranging from pure saline to pure contrast in the following increments: 0, 1/32, 1/16, 1/8, 1/4, 1/2, and 1. We investigated the dependency of the relaxation time, T1, on the concentration of the iodinated contrast agent in this phantom by acquiring an inversion recovery sequence with the following inversion times: 50, 100, 200, 400, 800, 1000, 3200, and 5000 ms on a 1.5 T clinical Achieva MRI system (Philips Healthcare, Best, The Netherlands).
Patient Study
We analyzed the presence or absence of postprocedural hemorrhage on subsequent CT and MR images statistically. Two experienced interventional neuroradiologists interpreted these studies. A positive study was defined as a new hyperdense area between 50 and 90 Hounsfield units (HUs) average on initial postprocedural CT and were classified as hemorrhage. A new hyperdense area less than 50 HU or greater than 90 HU average was classified as negative and deemed contrast extravasation.38,39 Equivocal interpretations of hemorrhage or contrast extravasation were deemed positive or negative depending on the working differential diagnosis for simplicity as determined by trained neuroradiologists’ interpretations. Of note, heterogeneity within the hyperdensity is categorized based on the density of the predominant finding. A positive study on MRI after 48 hours was defined as isointense on T1-weighted and hyperintense on T2-weighted images and was deemed hemorrhage. MRI interpretation was only restricted to T1- and T2-weighted sequences. Persistent hyperdensity, irrespective of change in size, on postprocedural CT images after 72 hours was considered a positive study and categorized as hemorrhage. True positive, false positive, true negative, false negative, predictive values, and likelihood ratio results were calculated using the software MedCalc, version 16.1 (Med-Calc, Microsoft Partner, Ostend, Belgium). Sensitivity, specificity, and accuracy were also obtained.
Results
In this retrospective single institution study, we initially assessed 500 eligible stroke patients through a database search in a 5-year time interval. Our stringent inclusion criteria yielded 10 patients who underwent
intra-arterial recanalization with subsequent CT without IV contrast done within 24 hours postprocedure,
MRI done within 48 hours, and
subsequent confirmatory CT done at least 72 hours afterward (Fig 1).
The overall sensitivity for the presence of postprocedure hemorrhage was 50%. In our patient population, we demonstrate a 50% (5/10) chance of diagnosing true postprocedural hemorrhage correctly when the CT done within 24 hours post-procedure and MRI done with 48 hours are positive and confirmed on noncontrast CT after 72 hours of the procedure. The overall specificity for the presence of postprocedural hemorrhage was also 50%. We show here a 50% (5/10) chance of diagnosing true contrast extravasation when the postprocedural CT and MRI are negative and confirmed on CT done 72 hours after the procedure. Positive predictive value was shown to be 60%. This suggests that patients with postprocedural CT within 24 hours and MRI done within 48 hours who were initially diagnosed with hemorrhage have a 60% rate of truly having a hemorrhage on confirmatory CT after 72 hours. Negative predictive value was deemed 40%. This indicates that patients initially diagnosed with contrast extravasation on CT and MRI have a 40% chances of truly having contrast extravasation on confirmatory noncontrast CT done within 72 hours. It is important to note that due to the small sample size, our confidence intervals have wide ranges.
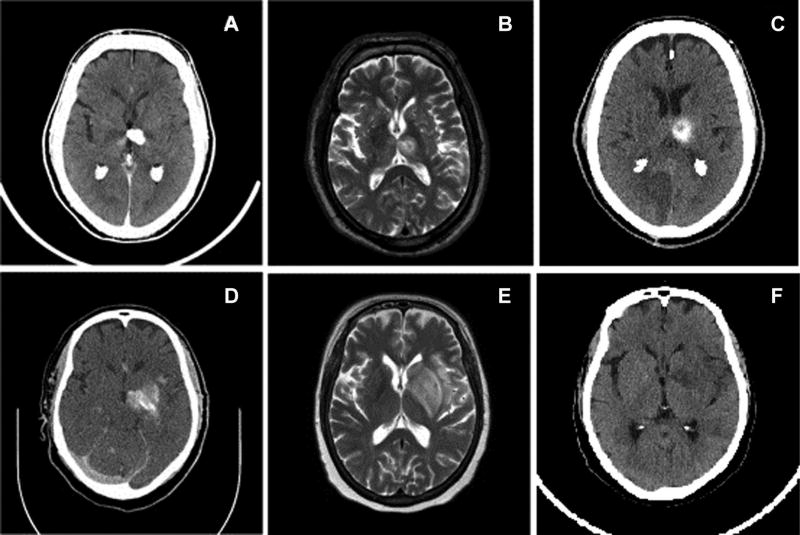
Figure 3 displays example images of both false positive and false negative studies in two stroke patients. The top left image (A) demonstrates a noncontrast head CT image done within 24 hours postprocedure with a well-marginated hyperdense lesion within the left thalamus and posterior basal ganglia measuring approximately 180 HU. Subsequent axial T2-weighted MR image done within 48 hours postprocedure as seen in top middle image (B) shows a rounded lesion with a slightly hyperintense core centered in the left thalamus with a peripheral rim of high signal and larger surrounding rim of intensity similar to that of the core. The following noncontrast CT done 72 hours in the top right image (C) after the procedure demonstrated a persistent hemorrhage. This is an example of a false negative case where the initial postprocedural head CT leads to a working differential diagnosis of contrast extravasation. Our study demonstrated a false negative ratio of 30%. The bottom row of Figure 3 is an example of a false negative study. The bottom left image (D) presents a noncontrast head CT done with 24 hours postprocedure with a heterogeneous hyperdense lesion primarily involving the left thalamus and basal ganglia with lateral extension near the insular cortex. The following axial T2-weighted MRI image done within 48 hours in the bottom middle (E) demonstrates a hyperintense lesion centered in the left basal ganglia primarily involving the striatum and insular cortex. The curvilinear central hyperdensity within the lesion was thought to be a mix of both hemorrhage and extravasation. However, because the majority of the lesion measured approximately 60 HU on average, this lesion was categorized as hemorrhage as per the criteria. The subsequent noncontrast CT done after 72 hours in the bottom right (F) shows contrast extravasation that was completely resorbed without residual hemorrhage. This is an example of a false positive case. Our study demonstrates a false positive rate of 20%. Postprocedural MR showed changes of the relaxation times T1 and T2. These changes are consistent with changes caused by iodine contrast agents and led to false positive postprocedural hemorrhage interpretations in 2 out of 10 patients in the MRI performed within 48 hours.44
Fig 3.
Representative images of two patients (patient 1 on top row, patient 2 on bottom row) with hyperdense lesions on CT postrecanalization procedure. CT image of patient 1, a 64-year-old male, with hyperdense lesion in the left thalamus with working diagnosis of contrast extravasation with hemorrhage not excluded (A). Axial T2-weighted MRI of patient 1 demonstrating a heterogeneously hyperintense lesion centered in the left thalamus (B). Seventy-two hour postprocedural image demonstrates persistent hemorrhage in the left thalamus (C). Patient 1 was deemed to have intracranial hemorrhage. CT image of patient 2, a 57-year-old female, with hyperdense lesion in left basal ganglia and insula with working diagnosis of hemorrhage with contrast extravasation not excluded (D). Axial T2-weighted MRI of patient 2 demonstrating a heterogeneously hyperintense lesion centered in the left basal ganglia, caudate nucleus, and insula (E). Seventy-two hour postprocedural CT image demonstrating complete resolution of the previously visualized hyperdensity within the left basal ganglia (F). Patient 2 was deemed to have contrast extravasation.
To demonstrate the effects of the iodinated CT contrast agent (Omnipaque 350) on the relaxation time T1, a phantom study was performed. Figure 4 displays a T1-weighted MR image of a dilution series in a phantom with different concentrations of the iodinated contrast agent. The concentrations are labeled in the figure as follows: In the center, 0 for physiological saline solution, 1/32, 1/16, 1/8, 1/4, 1/2, and 1 for pure contrast agent. The T1 shortening effect with increasing iodinated contrast agent solution is visible with brighter signal intensities with the brightest signal in the highest concentration. Our phantom study confirms the T1 shortening effects of iodinated contrast agents of a prior study of Jinkins et al.43
Fig 4.
A T1-weighted MR image of a dilution series of a phantom with different concentrations of an iodinated contrast agent (Omni-paque 350, GE Healthcare). The concentrations are labeled as follows: In the center, 0 for physiological saline solution, 1/32, 1/16, 1/8, 1/4, 1/2, and 1 for pure contrast agent. Brighter signal intensities in the vials with higher concentrations of the iodinated contract agent demonstrate T1 shortening effects.
Discussion
Hyperdense lesions on postprocedural CT after intra-arterial recanalization by thrombolysis or mechanical thrombectomy are common occurrences. However, a visualized hyperdense area confers increased risk of both hemorrhagic transformation as well as symptomatic postprocedural hemorrhage.1,11–17,28 Discerning whether these hyperdense lesions on CT are contrast extravasation or hemorrhage is crucial in stroke or trauma patients and can have significant clinical ramifications. Postprocedural iatrogenic hemorrhage has an incidence of between 10.9% and 15%12,29 and a mortality of up to 83% if symptomatic.12,29 Contrast extravasation is thought to be due to leakage from vessels into extracellular space from increased permeability of the blood brain barrier.30 Contrast effects can last up to 48 hours.31 Definite identification of extravasation requires frequent imaging for demonstration of eventual washout, while postprocedural hemorrhage is thought to persist for days to weeks.12,28
The main objective of this study was to report our experience with visualized hyperdense areas on immediate postprocedural CT as well as distinguishing the necessary time frame for a confirmatory MRI study. Moreover, we were attempting to address the use of additional contrast in confirmatory MRI studies as a possible confounding variable when assessing for contrast extravasation or hemorrhage. MRI has been utilized in limited capacity for determining the presence or absence of hemorrhage in post recanalization patients. While a number of studies have explored distinguishing hyperdense areas as contrast extravasation or hemorrhage,11–17 few have focused on how timing as well as use of gadolinium contrast in confirmatory imaging, such as MRI, can be modified. In our series, we demonstrated that the sensitivity and specificity of initial noncontrast CT and follow-up MRI done within 48 hours is 50%, respectively. Additionally, our phantom study corroborates our assertions that MRI done within 48 hours can be affected by residual angiographic iodinated contrast due to effects on T1 and T2 relaxation times as seen in previous studies.37 Overall, with sensitivity and specificity of 50%, our study demonstrates that our ability to detect postprocedural hemorrhage with CT and MRI within 48 hours is poor.
Another key component of our study is identifying the use of gadolinium contrast in the follow-up MRI done within 48 hours. Within our series, we encountered in 2 out of 10 patients this problem. Both were initially deemed equivocal on CT and were still considered equivocal on the follow-up MRI that was performed with gadolinium. This is crucial as it presents multiple diagnostic limitations. First, gadolinium contrast further obfuscates our initial concern of differentiating iodinated contrast extravasation from hemorrhage. As contrast is shown to wash out over time, the possibility of gadolinium contrast that also appears hyperdense on CT can mask an underlying hemorrhage.12,21–23 Moreover, this can lead to a higher false positive rate as gadolinium can persist, making the hyperdense area appear to be a hemorrhage when it is truly iodinated contrast extravasation.34–36 Second, reactions between iodinated contrast and gadolinium are virtually unknown, which could lead to further complications.34 Renal complications such as contrast-induced nephropathy or nephrogenic systemic fibrosis are well known for each contrast agent.34–36 Given the unknown of synergistic effects, this must be taken into consideration given the increased morbidity of systemic complications within stroke patients.35 This is a potential area of future research.
Limitations of our study include a small sample size. The small sample size is the result of a stringent inclusion criteria that necessitated a specific follow-up sequence of postprocedural CT within 24 hours, MRI within 48 hours, and confirmatory CT within 72 hours. Many of the patients did not qualify for our inclusion criteria because MRI was rarely utilized to follow up a hyperdense area found on initial postprocedural CT. In spite of the small sample size, our results of a 20% false positive and 30% false negative rate are similar to prior studies. The small sample size lead to wide confidence intervals for both our sensitivity and specificity values. Additionally, because of the small number, only differentiating existing postprocedural hemorrhage versus possible underlying contrast extravasation was investigated in the current study. Hemorrhagic transformation of a visualized hyperdense area was difficult to assess and presents a future research opportunity. We are optimistic that this current study has laid a foundation for future studies with more patients and incorporation of newer imaging modalities such as dual-energy CT (DECT) and advanced MR sequences such as susceptibility-weighted imaging (SWI).
Potential Future Economic Considerations—DECT versus MRI
DECT is now the gold standard for differentiating contrast extravasation versus iatrogenic hemorrhage. According to Phan et al, DECT has a sensitivity of nearly 100% and a specificity of 92.8%.17 Another study by Gupta et al concluded that DECT has a sensitivity of 100%, specificity of 91%, and accuracy of 93%.26 However, DECT also has some limitations. This modality has significantly improved the ability to differentiate extravasation from hemorrhage but has potentially increased radiation doses, is more expensive than single-source CT,17–19 and is not universally available in small hospitals when compared to both single-source conventional CT and MRI.20–23 Dinkel et al established that while DECT is accurate in 90.2% of cases, it is limited when there is beam hardening (4.2%), parenchymal calcifications (4.2%), motion (1.4%), and with saturation effect from iodine concentrations above 37 mg/dL.22 Postma et al also noted limitations with subtraction artifact when the HUs of vessels, calcified plaque, and bone are similar. DECT also has a longer postprocessing time relative to conventional CT.21
In addition to some of the technical limitations, DECT presents economic constraints as well. Certain studies asserted that DECT is currently not in widespread implementation and is only seen in academic institutions.17–19 Out of roughly 8,400 CT scanning sites in the country only 400 sites, fewer than 5%, have DECT capabilities.22 Reasons for lack of implementation include no set standardization and misconceptions about overall radiation dose. Cost is an additional significant disadvantage as DECT is more expensive than conventional CT, which limits its clinical use in smaller tertiary centers as well as community and rural settings. Generally, a CT with dual energy functionality costs between $1.6 and $2.5 million. In contrast, a conventional 64–80 slice CT costs roughly $550,000.32 There is also additional concern for increased radiation exposure, although this is thought to be controversial as some recent studies demonstrate equal or even lower doses compared to conventional CT. Others have also published significant decrease in radiation dose for conventional CT, which presents DECT as an unnecessary addition in current practice.17–22 Maintenance is another major concern as the cost of maintaining a DECT far exceeds its conventional counterpart.33 Finally, there is an added inertia as there is no additional reimbursement for interpretation of DECT compared to conventional CT.21,22
MRI still has a number of advantages when compared to CT. While it is expensive, it is considered a superior diagnostic tool compared to CT for a variety of pathologies.17,18,20,21 Specifically, SWI sequences have demonstrated benefit in diagnosing hemorrhage.19,38 SWI, in particular, has been shown to be a superior diagnostic tool in detecting different types of hemorrhage as well as distinguishing hemorrhage from calcification when compared to CT.9 Verma et al40 demonstrated the sensitivity of SWI to be 88% versus 75% when detecting subarachnoid hemorrhage. Additionally, the phase imaging component of SWI allows for greater differentiation between hemorrhage and calcifications due to the opposite phase values of calcium and heme as seen in Berberat et al.41 SWI is limited by its inability to quantify magnetic susceptibility.42 Overall, MRI also has the obvious benefit of no ionizing radiation exposure. Additionally, MRI is currently still more prevalent in clinical use as compared to DECT. In 2011, there were 10,395 MRI units and 13,530 total CT units (both conventional and DECT) in the United States.32,33 Given that 5% of the CTs seen today are DECT, it is a safe assumption that MRI has more mainstream clinical use ranging from academic to rural settings. Additionally, the absolute number of MRI machines has doubled in number from 2000 to 2011 within the United States furthering its mainstream usage compared to DECT.33
Conclusion
In this case series, we demonstrated the difficulties to distinguish contrast extravasation from hemorrhage in stroke patients post intra-arterial recanalization when finding a hyperdense area on initial postprocedural CT. Additionally, we show that a follow-up MRI done within 48 hours does not aid in distinguishing each entity when focusing on limited T1- and T2-weighted sequences. Our findings demonstrate that confirmatory MRI or CT should be done at least 72 hours after the recanalization procedure to account for residual effects of iodinated contrast administered during the procedure. We also conclude that a confirmatory MR should not be done with gadolinium contrast to prevent potential confounding effects of both the residual procedural contrast with the newly administered MR contrast as well as preventing possible complications from interactions. Moreover, with the increased risk of hemorrhagic transformation postprocedurally, the use of additional contrast would further obfuscate diagnosis. Our findings could lead to protocol changes in stroke patients in tertiary centers, community, and rural hospitals.
Acknowledgments
This study received no external funding.
Footnotes
Disclosure: The authors have no competing interest in this study.
References
- 1.Smith WS. Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke. Results of the multi Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trial, part I. AJNR AM J Neuroradiol. 2006;27:1177–82. [PMC free article] [PubMed] [Google Scholar]
- 2.Kuklina EV, Tong X, George M, et al. Epidemiology and prevention of stroke: a worldwide perspective. Expert Rev Neurother. 2012;12:199–208. doi: 10.1586/ern.11.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) National Center Health Statistics (NCHS) [Accessed February 3, 2015];Underlying Cause of Death 1999–2015. Available from CDC WONDER Online Database 2015. https://wonder.cdc.gov/wonder/help/ucd.html.
- 4.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 5.Hall MJ, Levant S, DeFrances CJ. Hospitalization for stroke in U.S. hospitals, 1989–2009 NCHS data brief, no. 95. Hyattsville, MD: National Center for Health Statistics; 2012. [PubMed] [Google Scholar]
- 6.Fang J, Keenan NL, Ayala C, et al. Awareness of stroke warning symptoms—13 states and the District of Columbia, 2005. MMWR. 2008;57:481–5. [PubMed] [Google Scholar]
- 7.Roberts TP, Chuang N, Roberts HC. Neuroimaging: do we really need new contrast agents for MRI? Eur J Radiol. 2000;34:166–78. doi: 10.1016/s0720-048x(00)00197-2. [DOI] [PubMed] [Google Scholar]
- 8.Trattnig S, Pinker K, Ba-Ssalamah A, et al. The optimal use of contrast agents at high field MRI. Eur Radiol. 2006;16:1280–7. doi: 10.1007/s00330-006-0154-0. [DOI] [PubMed] [Google Scholar]
- 9.Hermier M, Nighoghossian N. Contribution of susceptibility weighted imaging to acute stroke assessment. Stroke. 2004;35:1989–94. doi: 10.1161/01.STR.0000133341.74387.96. [DOI] [PubMed] [Google Scholar]
- 10.Yin N, Benavides S, Starkman S, et al. Autopsy findings of intracranial thrombectomy for acute ischemic stroke. Stroke. 2010;41:938–47. doi: 10.1161/STROKEAHA.109.576793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nikoubashman O, Reich A, Gindullis M, et al. Clinical significance of post-interventional cerebral hyperdensities after endovascular mechanical thrombectomy in acute ischaemic stroke. Neuroradiology. 2014;56:41–50. doi: 10.1007/s00234-013-1303-1. [DOI] [PubMed] [Google Scholar]
- 12.Greer DM, Koroshetz WJ, Cullen S, et al. Magnetic resonance imaging improves detection of intracerebral hemorrhage over computed tomography after intra-arterial thrombolysis. Stroke. 2004;35:491–5. doi: 10.1161/01.STR.0000114201.11353.C5. [DOI] [PubMed] [Google Scholar]
- 13.Shi ZS, Loh Y, Walker G. Endovascular thrombectomy for acute ischemic stroke in failed intravenous tissue plasminogen activator versus non-intravenous tissue plasminogen activator patients: revascularization and outcomes stratified by the site of arterial occlusions. Stroke. 2010;41:1185–92. doi: 10.1161/STROKEAHA.109.568451. [DOI] [PubMed] [Google Scholar]
- 14.Parrilla G, García-Villalba B, Espinosa de Rueda M, et al. Hemorrhage/contrast staining areas after mechanical intra-arterial thrombectomy in acute ischemic stroke: imaging findings and clinical significance. AJNR Am J Neuroradiol. 2012;33:1791–6. doi: 10.3174/ajnr.A3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kase CS, Furlan AJ, Wechsler LR, et al. Cerebral hemorrhage after intra-arterial thrombolysis for ischemic stroke: the PROACT II trial. Neurology. 2001;57:1603–10. doi: 10.1212/wnl.57.9.1603. [DOI] [PubMed] [Google Scholar]
- 16.Lummel N, Schulte-Altedorneburg G, Bernau C, et al. Hyperattenuated intracerebral lesions after mechanical recanalization in acute stroke. AJNR Am J Neuroradiol. 2014;35:345–51. doi: 10.3174/ajnr.A3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phan C, Yoo A, Hirsch J, et al. Differentiation of hemorrhage from iodinated contrast in different intracranial compartments using dual-energy head CT. AJNR Am J Neuroradiol. 2012;33:1088–94. doi: 10.3174/ajnr.A2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaza RK, Platt JF, Cohan RH, et al. Dual-energy CT with single-and dual-source scanners: current applications in evaluating the genitourinary tract. Radiographics. 2012;32:353–69. doi: 10.1148/rg.322115065. [DOI] [PubMed] [Google Scholar]
- 19.Liu YY, Cheng J, Zhang L, et al. A low-cost dual energy CT with sparse data. Tsinghua Sci Technol. 2014;19(2):184–94. [Google Scholar]
- 20.Kang MJ, Park CM, Lee CH, et al. Dual-energy CT: clinical applications in various pulmonary diseases. Radiographics. 2010;30:685–98. doi: 10.1148/rg.303095101. [DOI] [PubMed] [Google Scholar]
- 21.Postma A, Das M, Stadler A, et al. Dual-energy CT: what the neuroradiologist should know. Curr Radiol Rep. 2015;3:16. doi: 10.1007/s40134-015-0097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinkel J, Khalilzadeh O, Phan CM, et al. Technical limitations of dual-energy CT in neuroradiology: 30-month institutional experience and review of literature. J Neurointervent Surg. 2015;7:596–602. doi: 10.1136/neurintsurg-2014-011241. [DOI] [PubMed] [Google Scholar]
- 23.Halvorsen R. Which study when? Iodinated contrast-enhanced CT versus gadolinium-enhanced MR imaging. Radiology. 2008;249:9–15. doi: 10.1148/radiol.2491080593. [DOI] [PubMed] [Google Scholar]
- 24.MacDougall N, McVerry F, Baird S, et al. Iodinated contrast media and cerebral hemorrhage after intravenous thrombolysis. Stroke. 2011;42:2170–4. doi: 10.1161/STROKEAHA.111.618777. [DOI] [PubMed] [Google Scholar]
- 25.Ogura A, Hayakawa K, Mityati T, et al. Effects of iodinated contrast agent on diffusion weight magnetic resonance imaging. Acad Radiol. 2009;16:1196–200. doi: 10.1016/j.acra.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 26.Gupta R, Phan CM, Leidecker C, et al. Evaluation of dual-energy CT for differentiating intracerebral hemorrhage from iodinated contrast material staining. Radiology. 2010;257:205–11. doi: 10.1148/radiol.10091806. [DOI] [PubMed] [Google Scholar]
- 27.Eastwood J, Lev M, Provenzale J. Perfusion CT with iodinated contrast material. AJR Am J Roentgenol. 2003;180:3–12. doi: 10.2214/ajr.180.1.1800003. [DOI] [PubMed] [Google Scholar]
- 28.Nakano S, Iseda T, Kawano H, et al. Parenchymal hyperdensity on computed tomography after intra-arterial reperfusion therapy for acute middle cerebral artery occlusion. Stroke. 2001;32:2042–8. doi: 10.1161/hs0901.095602. [DOI] [PubMed] [Google Scholar]
- 29.Yoon W, Seo JJ, Kim JK, et al. Contrast enhancement and contrast extravasation on computed tomography after intra-arterial thrombolysis in patients with acute ischemic stroke. Stroke. 2004;35:876–81. doi: 10.1161/01.STR.0000120726.69501.74. [DOI] [PubMed] [Google Scholar]
- 30.Hergan K, Doringer W, Längle M, et al. Effects of iodinated contrast agents in MR imaging. Euro J Radiol. 1995;21:11–7. doi: 10.1016/0720-048x(95)00677-i. [DOI] [PubMed] [Google Scholar]
- 31.Lin SP, Brown JJ. MR contrast agents: physical and pharmacologic basics. J Magn Reson Imaging. 2007;25:884–99. doi: 10.1002/jmri.20955. [DOI] [PubMed] [Google Scholar]
- 32.Freiherr G. Do community hospitals need dual-energy CT? [Accessed May 2, 2016];Hitachi Medical. http://www.hitachimed.com/idc/groups/hitachimedical/documents/supportingdocumentpdf/Duel%20Energy%20White%20Paper.pdf.
- 33.Center for Disease Control and Prevention (CDC) Table 123 (page 1 of 2) [Accessed May 2, 2016];Number of magnetic resonance imaging (MRI) units and computed tomography (CT) scanners -Selected countries, selected years 1990–2009. Available at: http://www.cdc.gov/nchs/data/hus/2011/123.pdf.
- 34.OECD. Health at a Glance 2013: OECD Indicators. OECD Publishing; Paris: [Accessed May 2, 2016]. Medical technologies. [Google Scholar]
- 35.Golder W. Combined use of contrast media containing iodine and gadolinium for imaging, intervention. A hitherto widely ignored topic in radiological practice. Radiologe. 2012;52:167–72. doi: 10.1007/s00117-011-2279-7. [DOI] [PubMed] [Google Scholar]
- 36.Gierada D, Bae K. Gadolinium as a CT contrast agent: assessment in a porcine model. Radiology. 1999;210:829–34. doi: 10.1148/radiology.210.3.r99mr06829. [DOI] [PubMed] [Google Scholar]
- 37.Nikoubashman O, Jablawi F, Dekeyzer S, et al. MRI appearance of intracerebral iodinated contrast agents: is it possible to distinguish extravasated contrast agent from hemorrhage? AJNR Am J Neuroradiol. 2016;37:1418–21. doi: 10.3174/ajnr.A4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Payabvash S, Qureshi MH, Khan SM, et al. Differentiating intraparenchymal hemorrhage from contrast extravasation on post-procedural noncontrast CT scan in acute ischemic stroke patients undergoing endovascular treatment. Neuroradiology. 2014;56:737–44. doi: 10.1007/s00234-014-1381-8. [DOI] [PubMed] [Google Scholar]
- 39.Amans M, Cooke D, Vella M, et al. Contrast staining on CT after DSA in ischemic stroke patients progresses to infarction and rarely hemorrhages. Interv Neuroradiol. 2014;20:106–15. doi: 10.15274/INR-2014-10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verma RK, Kottke R, Andereggen L, et al. Detecting subarachnoid hemorrhage: comparison of combined FLAIR/SWI versus CT. Eur J Radiol. 2013;82:1539–45. doi: 10.1016/j.ejrad.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 41.Berberat J, Grobholz R, Boxheimer L, et al. Differentiation between calcification and hemorrhage in brain tumors using susceptibility-weighted imaging: a pilot study. AJR Am J Roentgenol. 2014;202:847–50. doi: 10.2214/AJR.13.10745. [DOI] [PubMed] [Google Scholar]
- 42.Liu C, Li W, Tong KA, et al. Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain. J Magn Reson Imaging. 2015;42:23–41. doi: 10.1002/jmri.24768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jinkins JR, Robinson JW, Sisk L, et al. Proton relaxation enhancement associated with iodinated contrast agents in MR imaging of the CNS. AJNR Am J Neuroradiol. 1992;13:19–27. [PMC free article] [PubMed] [Google Scholar]
- 44.Yedavalli V, Sammet S. Presented at the American Roengten Ray Society. New Orleans: May 1, 2017. The hidden bleed: contrast extravasation versus hemorrhage in post thrombectomy patients. [DOI] [PMC free article] [PubMed] [Google Scholar]