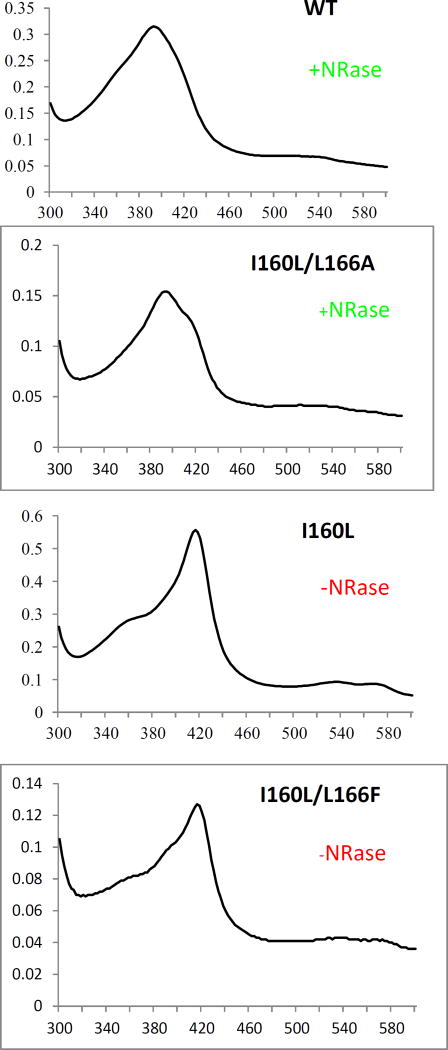
Figure 3.
Optical spectra of d-camphor (1)-bound WT CYP101A (top), I160L/L166A (second down), I160L (third down) and I160L/L166F. Horizontal axes are wavelengths (nm), and vertical axes are absorptions. The top two (WT and I160L/L166A) both reduce nitrobenzophenone 6 to 7a (+NRase) and have a significant high-spin component (λmax= 390 nm) with camphor bound. The bottom two exhibit little or no high-spin state with camphor bound (low-spin (λmax = 390 nm) and neither show reductase activity towards 6 (-NRase).