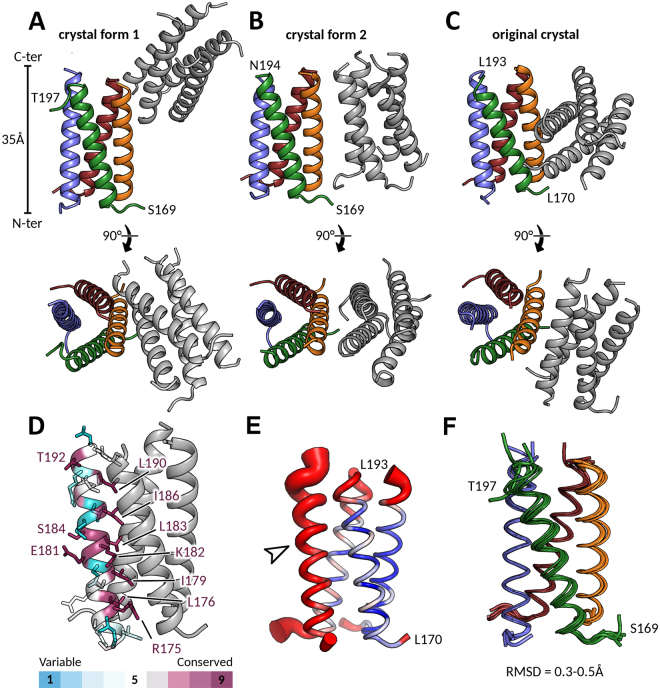
Figure 1.
Structures of HMPV Pcore from different crystal forms. A, B and C. Asymmetric units of two different Pcore crystal forms shown in side view and top view orientations. In both cases the asymmetric unit contained two coiled-coil Pcore tetramers (one depicted in grey and one coloured by chain). The structure in (A) is derived from the P21 crystal which diffracted to 1.6 Å, while the structure in (B) is derived from the P212121 crystal at 2.2 Å resolution. The previously published structure (PDB ID: 4BXT) is shown for comparison in (C) highlighting the distinct packing arrangements of each crystal. (D) The sequence conservation within Pneumoviridae members was mapped onto the Pcore structure and amino acids are coloured by conservation as indicated. For clarity, the side chains of only one of the four protomers are shown. Strictly conserved residues (coloured in deep purple) are explicitly labelled. E. B-factor putty representation of a single Pcore tetramer from crystal form 2. Red and thick regions of the putty represent high B-factors (~50 Å2), whilst thin and blue regions indicate low B-factors (~20 Å2). A solvent accessible helix displayed significantly higher B-factors that the rest of the coiled-coil (indicated by arrow). F. Structural superposition of six Pcore tetramers taken from crystal forms 1 and 2 and the previously published crystal (PDB ID: 4BXT).