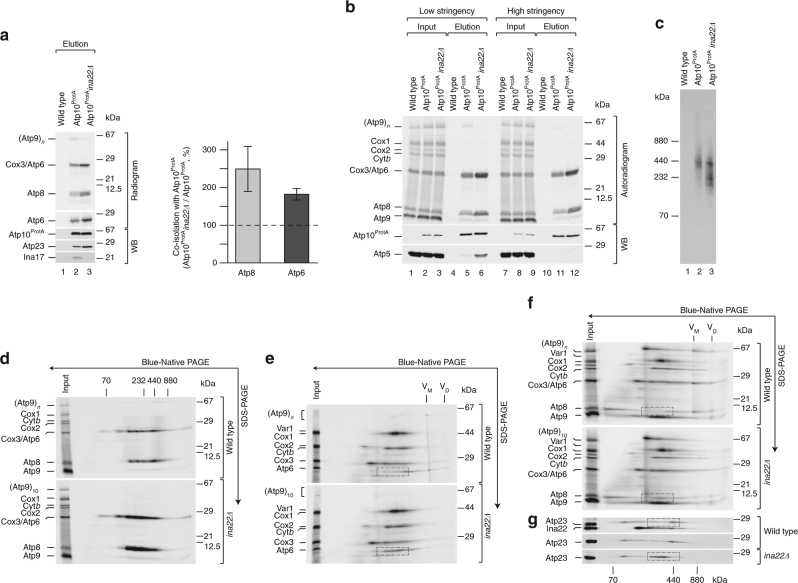
Fig. 5.
Atp10ProtA accumulates with Atp6/Atp8-containing assembly intermediate in ina22Δ. a Chloramphenicol-pretreated wild type, Atp10ProtA, and Atp10ProtA ina22Δ mitochondria were subjected to in organello labeling for 20 min and IgG-affinity chromatography. Input and elution fractions were analyzed by SDS-PAGE (for Atp8 quantification) or urea SDS-PAGE (for Atp6 quantification), followed by digital autoradiography and western blotting (WB). Amounts of Atp6 and Atp8, isolated from Atp10ProtA mitochondria, were set to 100%. Signals in the elution fraction were normalized to the amount of isolated Atp10ProtA, (n = 3, ±SEM). b Chloramphenicol-pretreated wild type, Atp10ProtA, and Atp10ProtA ina22Δ mitochondria were subjected to in organello labeling for 20 min and IgG-affinity chromatography under low-stringency conditions and high-stringency conditions. Input and elution fractions were analyzed by SDS-PAGE, digital autoradiography, and western blotting (WB). Input = 1% of elution. c Chloramphenicol-pretreated wild type, Atp10ProtA and Atp10ProtA ina22Δ mitochondria were subjected to in organello labeling for 20 min and IgG-affinity chromatography. Complexes were eluted natively and elution fractions were analyzed by BN-PAGE and digital autoradiography. d Part of the elution fraction of the experiment presented in b, was analyzed by BN-PAGE, second dimension SDS-PAGE and digital autoradiography. e, f Chloramphenicol-pretreated wild type and ina22Δ mitochondria were subjected to in organello labeling for 15 min. Protein complexes were analyzed by BN-PAGE and second dimension urea SDS-PAGE (e) or SDS-PAGE (f) followed by digital autoradiography. Dashed boxes show Atp6/Atp8-containing assembly intermediates. g Chloramphenicol-pretreated wild type and ina22Δ mitochondria were analyzed by BN-PAGE, second dimension SDS-PAGE, and western blotting with the indicated antibodies. Dashed boxes show Atp23-containing complexes