Abstract
Pancreatic ductal adenocarcinoma (PDAC) is the most lethal malignancy known, with an extremely poor prognosis due to the lack of an efficient diagnostic scheme and no radical treatment option, except surgery. Therefore, understanding the pathophysiology of, and finding a novel biomarker to detect, PDAC should be prioritized. We observed an increase in mRNA expression of the cysteine protease inhibitor cystatin A (CSTA) in CD4+ T cells in peripheral blood cells of nine patients with PDAC, compared with the expression in seven healthy volunteers. Moreover, we confirmed significantly higher CSTA mRNA expression in a larger cohort of 41 patients with PDAC compared with that in 20 healthy volunteers. Correspondingly, the serum CSTA concentrations in 36 patients with PDAC were higher than those in 37 healthy volunteers, and this increase was correlated with PDAC clinical stage. Furthermore, the expression of CSTA and cathepsin B, which is a lysosomal cysteine protease inhibited by CSTA, was observed in tumor tissues and tumor‐infiltrating immune cells in 20 surgically resected PDAC tissues by immunohistochemical staining. Expression of CSTA was detected in some tumor tissues and many tumor‐infiltrating immune cells. Cathepsin B expression was also observed in most tumor tissues and tumor‐infiltrating immune cells. In conclusion, CSTA and its substrate cathepsin B are involved in PDAC‐related inflammation. The increment of CSTA expression in peripheral blood of patients with PDAC may have a potential role as a PDAC immunopathologic biomarker.
Keywords: Cathepsin B, CD4+ T cell, cystatin A, pancreatic cancer, peripheral blood
Pancreatic ductal adenocarcinoma (PDAC) is the most lethal malignancy known, with an extremely poor prognosis and a 5‐year survival rate of <5% worldwide.1 Surgical resection is the only treatment available that can achieve a complete cure; however, only 15–20% of patients are diagnosed in the early (i.e., operable) stages.2, 3 Chemotherapy is an alternative for unresectable PDAC; however, any survival benefit is limited. Therefore, it is extremely important to establish novel diagnostic biomarkers for PDAC, indicating curative surgical treatment.4
Peripheral blood (PB) contains a variety of immune‐mediating cells, such as neutrophils, lymphocytes, and monocytes, which are involved in the host immune defense system and respond to various diseases, such as viral infection, metabolic disease, and cancers.5, 6, 7, 8 Peripheral blood cells alter their gene expression profile in response to various diseases9, 10 including PDAC.11 We previously investigated the gene expression features of patients with digestive cancers12 and found that these cancers affect the expression of the cysteine protease inhibitor cystatin A (CSTA) in PB cells.
In this study, we report that CSTA expression was upregulated in the CD4+ cells of patients with PDAC. We also observed that the serum CSTA level was higher in patients with PDAC compared with healthy controls. An immunohistochemical staining analysis of surgically resected PDAC tissues showed that CSTA and the lysosomal cysteine protease cathepsin B, which is inhibited by CSTA, were expressed in tumor tissues and tumor‐infiltrating immune cells. Thus, CSTA and cathepsin B play an important role in the local cancer tissues and PB of patients with PDAC.
Materials and Methods
Patients
Nine patients with PDAC (male : female, 8:1; age, 70.8 ± 8.9 years) and seven healthy volunteers (male:female, 4:3; age, 61.0 ± 3.7 years) were subject to gene expression analysis of CD4+ T cells, CD8+ T cells, CD14+ monocytes, CD15+ neutrophils, and CD19+ B cells in PB (Table 1). The groups were not different with respect to other clinical parameters. Another, larger cohort of 41 patients with PDAC (male:female, 30:11; age, 73.3 ± 11.3 years) and 20 healthy volunteers (male:female, 6:14; age, 61.1 ± 9.6 years) were also enrolled. In addition, serum CSTA concentrations in 36 patients with PDAC (male:female, 27:9; age, 71.2 ± 9.8 years) and 37 healthy volunteers (male:female, 18:19; age, 62.4 ± 7.7 years) were measured (Table 2). The groups were not significantly different with respect to other clinical parameters. In terms of the clinical background of PDAC patients for the gene expression analysis (Table 1) and serum CSTA concentration analysis, the frequency of patients with distant metastasis or stage IV disease was 77.8% and 63.8%, respectively; the difference was not statistically significant. The ratio of patients with tumor marker carcinoembryonic antigen (CEA) ≥10 ng/mL in Table 1 (66.7%; 6/9 patients) was significantly higher than that in Table 2 (22.2%; 8/36 patients; P = 0.0297). Other clinical parameters such as age, white blood cell count, frequency of peripheral lymphocytes and monocytes, hemoglobin, and serum concentration of CA19‐9 were not significantly different between these two groups.
Table 1.
Characteristics of gene expression in study subjects
| PDAC patients (n = 9) | Healthy volunteers (n = 7) | P‐value | |
|---|---|---|---|
| Age, years | 70.8 ± 8.9 | 61.0 ± 3.7 | <0.001 |
| Gender, male / female | 8/1 | 4/3 | NS |
| White blood cell count,/μL | 7500 ± 1600 | 6300 ± 1700 | NS |
| Lymphocyte cell count, % | 21.2 ± 4.7 | 20.7 ± 4.5 | NS |
| Monocyte cell count, % | 8.7 ± 2.4 | 7.2 ± 2.2 | NS |
| Hemoglobin, g/dL | 12.1 ± 2.3 | 13.2 ± 1.2 | NS |
| CEA, ng/mL, <5 / ≥5, <10 / ≥10, <30 / ≤30 | 2/1/2/4 | NA | |
| CA19‐9, U/dL, <37 / ≥37, <200 / ≤200, <400 / ≤400 | 1/2/1/5 | NA | |
| TNM stage, I / II / III / IV | 0/2/0/7 | NA | |
| Distant metastasis, + / − | 7/2 | NA |
Data are expressed as mean ± SD. CA19‐9, cancer antigen 19‐9; CEA, carcinoembryonic antigen; NA, not applicable; NS, not significant; PDAC, pancreatic ductal adenocarcinoma.
Table 2.
Characteristics of study subjects for serum cystatin A expression
| PDAC patients (n = 36) | Healthy volunteers (n = 37) | P‐value | |
|---|---|---|---|
| Age, years | 71.2 ± 9.8 | 62.4 ± 7.7 | <0.001 |
| Gender, male / female | 27/9 | 18/19 | NS |
| White blood cell count,/μL | 5800 ± 1700 | 5600 ± 1900 | NS |
| Lymphocyte cell count, % | 24.2 ± 3.8 | 22.7 ± 3.5 | NS |
| Monocyte cell count, % | 5.4 ± 1.9 | 6.2 ± 2.1 | NS |
| Hemoglobin, g/dL | 13.5 ± 1.4 | 13.8 ± 1.6 | NS |
| CEA, ng/mL, <5 / ≥5, <10 / ≥10, <30 / ≤30 | 24/4/5/3 | NA | |
| CA19‐9, U/dL, <37 / ≥37, <200 / ≤200, <400 / ≤400 | 14/6/1/15 | NA | |
| TNM stage, I / II / III / IV | 1/1/11/23 | NA | |
| Distant metastasis, + / − | 13/23 | NA |
Data are expressed as mean ± SD. CA19‐9, cancer antigen 19‐9; CEA, carcinoembryonic antigen; NA, not applicable; NS, not significant; PDAC, pancreatic ductal adenocarcinoma.
Clinical tumor stage was assessed using the TNM staging system for pancreatic carcinoma of the UICC (7th edition). Informed consent was obtained from each subject. This study was approved by the institutional ethics committee and carried out in accordance with the Declaration of Helsinki.
Isolation of subpopulations of PB cells and flow cytometry
Peripheral blood cells were isolated from fresh heparinized venous blood using ACK lysing buffer in accordance with the manufacturer's protocol (Lonza, Basel, Switzerland). The subpopulations of PB cells were isolated using a magnetic cell sorting system, bead‐labeled anti‐CD4, anti‐CD8, anti‐CD14, anti‐CD15, and anti‐CD19 antibodies (Miltenyi, Cologne, Germany), and a magnet column (Miltenyi) according to the manufacturer's protocol (Miltenyi Biotec, Bergisch Gladbach, Germany). Cell purity was confirmed to be >95% by flow cytometric analysis using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA).
Quantitative RT‐PCR analysis
Total RNA was isolated from cells using a microRNA isolation kit (Stratagene, La Jolla, CA, USA) and was reverse‐transcribed using 1 μg oligo(dT) primer and SuperScript II Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. Primer pairs and probes for CSTA, T‐bet, Foxp3, γ‐interferon (IFN‐γ), transforming growth factor‐β (TGF‐β), and β‐actin (Applied Biosystems, Foster City, CA, USA) were used for the mRNA expression analysis with the ABI PRISM 7900 HT Sequence Detection System (Applied Biosystems). The relative gene expression levels were calculated with the method using β‐actin as the control gene.11
Serum CSTA concentrations
Serum CSTA concentrations were measured with a human ELISA kit (Biocompare, San Francisco, CA, USA) according to the manufacturer's protocol. The detection range was 0.313–20 ng/mL.
Immunohistochemical analysis of surgically resected pancreatic cancer tissues
For immunohistochemical staining, 4‐μm tissue block sections were incubated overnight with rabbit anti‐human CSTA mAb (clone EPR6941, dilution 1:500; Abcam, Cambridge, UK), mouse anti‐human monoclonal cathepsin B antibody (clone CA10, dilution 1:200; Abcam), mouse anti‐human monoclonal T‐bet antibody (clone ERP9301, dilution 1:200; Abcam), mouse anti‐human monoclonal Foxp3 antibody (clone 52B83, dilution 1:200; Abcam), mouse anti‐human monoclonal IFN‐γ antibody (clone IFNG/466, dilution 1:200; Abcam), mouse anti‐human monoclonal tumor necrosis factor‐α (TNF‐α) antibody (clone 52B83, dilution 1:200; Abcam), mouse anti‐human monoclonal TGF‐β antibody (clone TB21, dilution 1:200; Abcam), mouse anti‐human monoclonal interleukin (IL)‐6 antibody (clone 10C12, dilution 1:50; Leica Biosystems, Newcastle, UK) or rabbit anti‐human polyclonal IL‐1β antibody (clone H‐153, dilution 1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA) after heat‐induced antigen retrieval. Following incubation with the antibody, the samples were incubated at room temperature for 1 h with anti‐mouse immunoglobulins conjugated to a peroxidase‐labeled dextran polymer (Simple Staining Kit; Nichirei, Tokyo, Japan). After the benzidine reaction, the sections were lightly counterstained with hematoxylin.
Serum cytokine and chemokine concentrations in PDAC patients
Serum concentrations of cytokines/chemokines were measured using a Multiplex Bead Immunoassay kit and Human Cytokine 27‐Plex Panel (Invitrogen) including IFN‐γ, IL‐6, IL‐1β, and TNF‐α, according to the manufacturer's protocol. Serum was reserved from six PDAC patients with positive CSTA expression and nine PDAC patients with negative CSTA expression.
Statistical analysis
Data are expressed as mean ± SE. The Mann–Whitney U‐test was used to detect differences between the two groups. A P‐value <0.05 was considered significant. Pearson correlation coefficients and multiple regression analysis were used to analyze correlations.
Results
Cystatin A expression and concentration were elevated in CD4+ T cells and sera of patients with PDAC
We isolated CD4+ T cell, CD8+ T cell, CD14+ monocyte, CD15+ neutrophil, and CD19+ B cell fractions from whole PB cells, and examined CSTA gene expression. CSTA expression in the CD4+ T cells of nine patients with PDAC was significantly higher than in those of healthy volunteers, but no differences were observed for CD8+ T cells or CD19+ B cells, nor CD14+ monocytes or CD15+ neutrophils, both of which were originally abundant with CSTA expression (Fig. 1a). We also assessed CSTA gene expression in CD4+ T cells in another large cohort, consisting of 41 patients with PDAC and 20 healthy volunteers. Gene expression of CSTA in the CD4+ T cells of patients with PDAC was significantly higher than that of healthy volunteers (Fig. 1b). CSTA expression was not significantly correlated with age of the patients with PDAC (R = −0.207). To assess whether enhanced CSTA expression in peripheral CD4+ T cells of PDAC patients was related to the specific subset of CD4+ T cells, such as antitumor helper T cells (Th1) or regulatory T cells (T‐reg), which are characterized by inhibition of antitumor immunity, we measured the mRNA expression of molecules including transcriptional factors (T‐bet for Th1, and Foxp3 for T‐reg) and cytokines (IFN‐γ for Th1, and TGF‐β for T‐reg) in CD4+ T cells of PDAC patients by quantitative RT‐PCR, followed by correlation analysis. We did not observe any significant correlation with mRNA expression of CSTA and genes related to either antitumor helper Th1 or T‐reg subsets, or these cytokines (Fig. S1). Thus, CD4+ T cells expressing CSTA in PB was not categorized into any specific conventional CD4+ subset.
Figure 1.
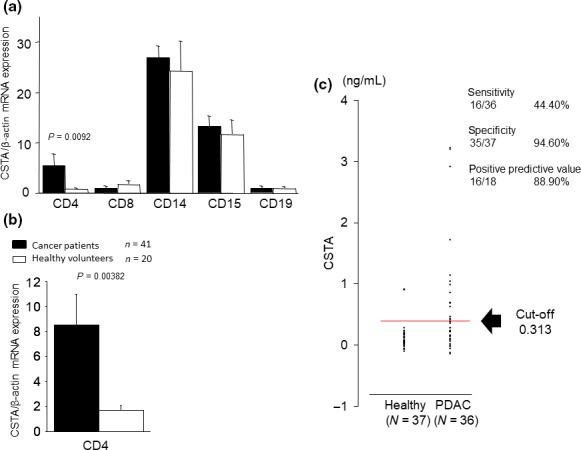
Cysteine protease inhibitor cystatin A (CSTA) expression in peripheral blood cells and serum CSTA concentrations in patients with pancreatic ductal adenocarcinoma (PDAC). (a) CSTA expression was upregulated in the CD4 + T cells of nine patients with PDAC, but no differences in expression were detected in CD8+ T cells, CD14+ monocytes, CD15+ neutrophils, or CD19+ B cells, compared with seven healthy volunteers. (b) CSTA expression was upregulated in the CD4+ T cells of 41 patients with PDAC compared with 20 healthy volunteers. (c) Serum CSTA concentrations in 36 patients with PDAC were higher compared with those of 37 healthy volunteers. Detection sensitivity was 16/36 (44.4%), and specificity was 35/37 (94.6%). Moreover, the positive predictive value was 16/18 (88.9%) and the negative predictive value was 35/55 (63.6%).
Next, we measured serum CSTA concentrations in 36 patients with PDAC and 37 healthy volunteers. Serum CSTA concentration was significantly increased in patients with PDAC (Fig. 1c). When we defined the cut‐off value as 0.313 ng/mL, which is the minimal detection limit of the ELISA kit, detection sensitivity was 16/36 (44.4%), and specificity was 35/37 (94.6%). The positive predictive value was 16/18 (88.9%) in these participants. These results show enhanced expression of CSTA in PB CD4+ cells and elevated concentrations of CSTA in the sera of patients with PDAC.
We also measured the serum cytokine concentration of 15 PDAC patients: an increment of CSTA expression in six patients and nine PDAC patients without elevation. Serum concentration of pro‐inflammatory cytokines IFN‐γ (Fig. S2A), TNF‐α (Fig. S2B), IL‐6 (Fig. S2C), and IL‐1β (Fig. S2D) was relatively high in PDAC patients with high expression of CSTA in sera compared to those without CSTA increment. Correlation analysis showed that serum concentrations of IFN‐γ (Fig. S2A), TNF‐β (Fig. S2B), and IL‐1β (Fig. S2D) were significantly correlated with that of CSTA in sera of PDAC patients. These data suggest that the elevated pro‐inflammatory cytokine concentrations were related to the elevated concentration of CSTA in sera of PDAC patients.
Comparison of serum CSTA concentration and clinical parameters in patients with PDAC
We further assessed the associations between serum CSTA concentration and several clinical parameters. Gender (Fig. 2a) and the presence of distant metastasis were not associated with serum CSTA concentration (Fig. 2b). However, serum CSTA positivity was significantly correlated with advanced clinical stage (III–IV; Fig. 2c). We also assessed serum concentrations of the tumor markers CEA and CA19‐9 in patients with PDAC according to CEA (<5 / ≥5, <10 / ≥10, <30 / ≥30 ng/mL; n = 24/4/5/3) and CA19‐9 level (<37 / ≥37, <200 / ≥200, <400 / ≥400 U/mL; n = 14/6/1/15). No association was detected between CEA level (Fig. 2d) and serum CSTA positivity. However, CA19‐9 level ≥400 U/mL was correlated with CSTA positivity (Fig. 2e), as well as clinical stage (data not shown).
Figure 2.
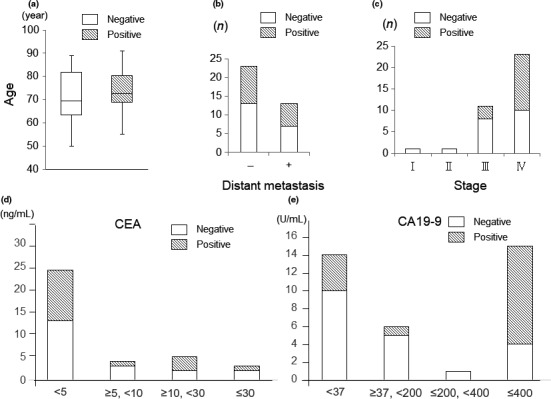
Correlation between serum cystatin A (CSTA) concentration and several clinical parameters in patients with pancreatic ductal adenocarcinoma. No association was found between CSTA concentration and gender (a) or the presence of distant metastasis (b). (c) The serum CSTA concentration was increased in patients with stage IV disease. (d) There was no correlation between the carcinoembryonic antigen (CEA) value and the serum CSTA concentration. (e) A significant correlation was observed between the level of cancer antigen 19‐9 (CA19‐9; ≤400 / <400 U/mL) and the serum CSTA concentration.
Expression of CSTA and cathepsin B in tumor tissues and tumor‐infiltrating immune cells
To further investigate the features of CSTA‐related pathophysiology in patients with PDAC, the expression of CSTA and cathepsin B, which is a lysosomal cysteine protease, was evaluated in the tumor tissues and tumor‐infiltrating immune cells of 20 surgically resected PDAC tissues by immunohistochemical staining (Table 3). Cystatin A expression either in tumor cells or tumor‐infiltrating immune cells was found in 16 of 20 patients (80%) (Table 3). Tumor‐infiltrating immune cells expressing CSTA, mostly neutrophils, were found in 15 of 20 patients; among them, 11 patients (73%) were not associated with tumor cells expressing CSTA (Table 3). In tumor cells CSTA expression was found in 5 of 20 patients (25%) (Fig. 3a,b, Table 3), and cathepsin B in 15 patients (75%) (Fig. 3c,d, Table 3). As for tumor‐infiltrating immune cells, CSTA expression was not or weakly founded in mostly neutrophils (Fig. 4a,b, Table 3), while cathepsin B, were found in all 20 PDAC patients (100%) in mostly macrophages (Fig. 4c,d, Table 3). Thus, CSTA expression in PDAC tissues was found predominantly in tumor‐infiltrating immune cells, and cathepsin B expression was found both in tumor cells and tumor‐infiltrating immune cells.
Table 3.
Cystatin A and cathepsin B expression in 20 resected specimens of pancreatic ductal adenocarcinoma tumor tissue and infiltrating inflammatory cells
| Case no. | Age, years | Gender | Degree of inflammation | Stage | T category | N category | CRP, mg/dL | CEA, ng/mL | CA19‐9, U/mL | Cathepsin B | Cathepsin B | Cathepsin B | Cystatin A | Cystatin A | Cystatin A |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tumor | Ductal epithelium | TIIC | Tumor | Ductal epithelium | TIIC | ||||||||||
| 1 | 71 | F | Mild | II B | 3 | 1 | 0.2 | 2.2 | 157 | + | − | >100 | − | − | 0 |
| 2 | 57 | M | Moderate | II B | 3 | 1 | 0.1 | 4.3 | 293 | + | − | >100 | − | − | 0 |
| 3 | 61 | F | Severe | II B | 1 | 1 | 0.0 | 2.5 | 17 | + | + | >100 | − | − | >100 |
| 4 | 54 | F | Mild | II B | 3 | 1 | 0.1 | <2.0 | 54 | + | − | 15 | + | − | <10 |
| 5 | 70 | F | Mild | II B | 3 | 1 | 0.0 | <2.0 | 90 | + | − | 62 | − | − | >100 |
| 6 | 66 | M | Moderate | II B | 3 | 1 | 0.1 | 2.8 | 56 | − | − | >100 | + | − | >100 |
| 7 | 60 | F | Moderate | II B | 3 | 1 | 0.5 | 30.1 | 649 | + | − | 35 | − | − | 45 |
| 8 | 78 | M | Moderate | II A | 3 | 0 | 0.5 | 4.5 | 23 | + | − | <10 | + | − | <10 |
| 9 | 77 | M | Moderate | II A | 3 | 0 | 0.5 | <2.0 | 187 | + | − | 39 | − | − | <10 |
| 10 | 57 | M | Moderate | II B | 3 | 1 | 0.1 | <2.0 | 402 | − | − | 25 | − | − | 14 |
| 11 | 65 | M | Moderate | II B | 3 | 1 | 0.9 | 4.2 | 292 | − | − | 31 | + | − | <10 |
| 12 | 68 | F | Severe | II B | 3 | 1 | 2.7 | <2.0 | 50 | + | − | >100 | − | − | 45 |
| 13 | 62 | M | Mild | II A | 3 | 0 | 0.0 | <2.0 | 57 | + | + | 55 | − | − | 38 |
| 14 | 65 | M | Moderate | II A | 3 | 0 | 0.0 | 3.4 | 184 | + | − | 18 | − | − | <10 |
| 15 | 59 | F | Mild | II A | 3 | 0 | 0.1 | <2.0 | 18 | − | − | 33 | − | − | 13 |
| 16 | 66 | M | Moderate | II A | 3 | 0 | 0.1 | 3.2 | 9 | + | − | <10 | − | − | 0 |
| 17 | 70 | M | Moderate | II B | 3 | 1 | 0.1 | 1.5 | 85 | + | − | 22 | + | − | 0 |
| 18 | 57 | F | Moderate | II A | 3 | 0 | 0.1 | 4.9 | 7 | + | − | 43 | − | − | 0 |
| 19 | 63 | M | Mild | I B | 2 | 0 | 0.9 | 2.5 | <1 | + | + | 35 | − | − | 46 |
| 20 | 64 | F | Moderate | II A | 3 | 0 | 0.0 | 2.7 | 33 | − | − | >100 | − | − | <10 |
CA19‐9, cancer antigen 19‐9; CEA, carcinoembryonic antigen; CRP, C‐reactive protein; F, female; M, male; TIIC, Tumor infiltrating immune cells.
Figure 3.
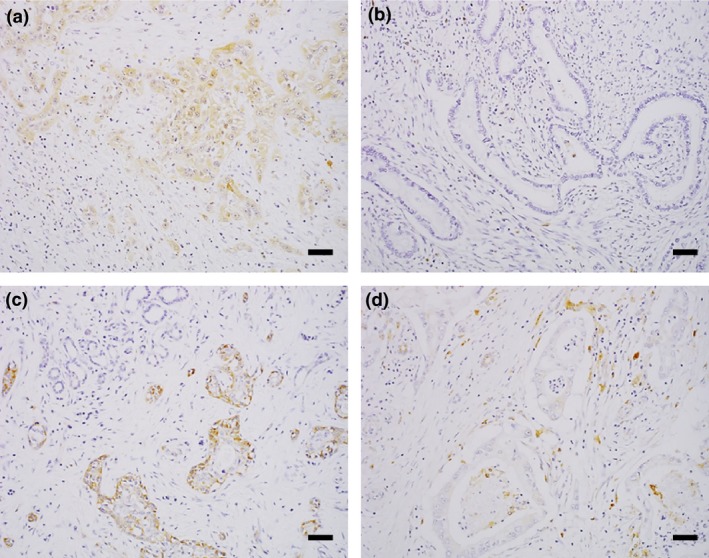
Cystatin A (CSTA) and cathepsin B expression in tumor tissues from patients with pancreatic ductal adenocarcinoma. (a, b) Representative images showing the presence (patient no. 11) (a) and absence (patient no. 13) (b) of CSTA in tumor tissue. (c, d) Representative images showing the presence (patient no. 8) (c) and absence (patient no. 15) (d) of cathepsin B in tumor tissue. Scale bar = 100 μm.
Figure 4.
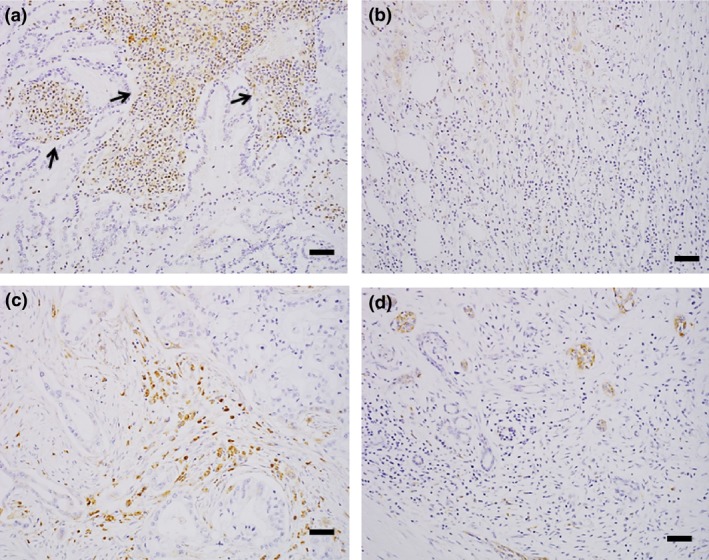
Cystatin A (CSTA) and cathepsin B expression in tumor‐infiltrating immune cells from patients with pancreatic ductal adenocarcinoma. (a, b) Representative images of the substantial presence (patient no. 6) (a) and minimal presence (patient no. 11) (b) of CSTA in tumor‐infiltrating immune cells. CSTA‐positive infiltrating cells (arrows) are mainly neutrophils. (c, d) Representative images of the substantial presence (patient no. 6) (c) and minimal presence (patient no. 8) (d) of cathepsin B in tumor‐infiltrating immune cells. Cathepsin B‐positive cells are mainly macrophages. Scale bar = 100 μm.
We also undertook immunohistochemistry of PDAC tissues using a multicolor assay for transcriptional factors T‐bet (Fig. S3A) and Foxp3 (Fig. S3B), and cytokines IFN‐γ (Fig. S3C), TGF‐β (Fig. S3D), TNF‐α (Fig. S3E), IL‐6 (Fig. S3F), and IL‐1β (Fig. S3G), together with CSTA. We found a substantial number of tumor‐infiltrating immune cells expressing IFN‐γ and TGF‐β (Fig. S3C,D); however, we did not find tumor‐infiltrating immune cells expressing these cytokines concomitantly with CSTA, except that a very few cells expressing CSTA and IFN‐γ were detected. We did not find any tumor‐infiltrating immune cells expressing TNF‐α (Fig. S3E), and rarely found cells expressing IL‐6 (Fig. S3F) and IL‐1β (Fig. S3G). Taken together, CSTA‐expressing tumor‐infiltrating immune cells were involved in PDAC tissues, which were independent from cells expressing cytokines including profibrogenic TGF‐β, not TNF‐α, suggestive of the fibrotic condition of PDAC tissues.13
Discussion
In this study, we identified upregulated expression of CSTA in CD4+ T cells of PB, as well as elevated serum concentrations of CSTA, in patients with PDAC. The increase in serum concentrations of CSTA was correlated with clinical stage. Cystatin A expression was observed mainly in tumor‐infiltrating immune cells of surgically resected PDAC tissues, particularly neutrophils, and cathepsin B was observed in tumor cells as well as tumor‐infiltrating immune cells, particularly macrophages.
Cystatin A is a member of the cystatin superfamily of cytoplasmic cysteine protease inhibitors. Cathepsin B belongs to the human cysteine protease cathepsin family, which has 11 members,14 and is generally expressed in epithelial cells, immune‐mediating cells, and lymphoid tissue.15 It is a key acid hydrolase within the lysosome and represents one of the principal effectors of protein catabolism and autophagy.16, 17, 18 Cystatin A inhibits the enzymatic activity of cathepsin B.
The role of cathepsin B expression in tumor cells is controversial, and has been reported to be both progressive and regressive.19, 20, 21, 22 Cathepsin B expression in patients with PDAC is related to prognosis and recurrence,23, 24, 25 although the mechanism remains to be elucidated. Cystatin A expression has been observed in tumor tissues, such as breast,26 head and neck,27 and lung28 cancers, as well as hepatocellular carcinoma.29 In the current study, cathepsin B was expressed in tumor cells, as well as tumor‐infiltrating immune cells, especially macrophages, in PDAC tissues. In contrast, CSTA expression was observed mainly in tumor‐infiltrating immune cells rather than tumor cells, which represents a different pathological feature to those of other cancer types.26 In this context, CSTA could affect cathepsin during antigen‐presenting processes involving macrophages and dendritic cells,30, 31 resulting in an altered immune response of the host to local tumor cells.32, 33 The local pathological conditions mediated by cathepsin and CSTA in tumor cells and tumor‐infiltrating immune cells affect tumor progression. The responses of cathepsin and the CSTA complex affect tumor progression and metastasis in laryngeal34 and breast cancers.26 Furthermore, tumor cells overexpressing CSTA showed a reduced capacity for lung or bone metastasis in a human esophageal squamous cell carcinoma xenograft model and a syngeneic mouse model of mammary gland tumorigenesis, respectively, suggesting that CSTA could act as a tumor metastasis suppressor in this complex.35, 36 Previously, there were some reports that the expression ratio of cathepsin B and cystatin families, such as cystatin B or cystatin C, in sera were significantly correlated with presence of cancer, prognosis, or lymph node metastasis in patients with colorectal cancer,37 esophageal cancer,38 and cholangiocarcinoma.39 Considering these, the ultimate consequences of the presence of CSTA‐expressing cells and cathepsin‐expressing cells in PDAC tissues, elevated CSTA concentration in sera, and increased expression of CSTA in peripheral CD4+ T cells of PDAC patients should be further investigated.40
Intriguingly, we also observed that CSTA expression in the PB CD4+ T cells of patients with PDAC was higher than in those of healthy volunteers. In addition, serum CSTA concentrations in patients with PDAC were higher than those of healthy volunteers. Considering the involvement of cathepsin B and CSTA in tumor cells and tumor‐infiltrating immune cells, it is possible that PB reflects CSTA activity in the local tumor microenvironment, as we previously reported the PB biological signature features of gene expression under inflammatory conditions in local cancer tissues.9 Thus, upregulated expression of CSTA in CD4+ T cells, and increased serum CSTA concentration in PB, is presumably a reflection of the local PDAC tumor environment involving cathepsin B and CSTA. The characteristics of peripheral CD4+ T cells expressing CSTA in PB should be further investigated, as they were not indicative of the conventional antitumor immune Th1 cells, nor T‐reg, which is inhibitory to antitumor immunity. In addition, most tumor‐infiltrating immune cells expressing CSTA were not relevant to CD4+ T cells. Further investigations are needed to disclose details of the roles of CSTA for cancer immunity.
In conclusion, the current study indicates that CSTA expression is involved in the PDAC inflammatory condition in local tumor tissues, including with respect to the environment associated with cathepsin B expression, as well as PB. Further investigations are needed to elucidate the significance of the CSTA–cathepsin axis in the pathophysiology of PDAC, to aid development of novel diagnostic and treatment approaches.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. Gene expression of CSTA, transcriptional factors, and cytokines in peripheral CD4+ T cells of patients with pancreatic ductal adenocarcinoma.
Fig. S2. Serum concentrations of cytokines and cystatin A (CSTA) in patients with pancreatic ductal adenocarcinoma (PDAC).
Fig. S3. Immunohistochemical analysis of with pancreatic ductal adenocarcinoma tissues for transcriptional factors and cytokines related to CD4+ T subsets T‐bet (A), Foxp3 (B), γ‐interferon (IFN‐γ) (C), and transforming growth factor‐β (TGF‐β) (D), as well as cytokines tumor necrosis factor‐α (TNF‐α) (E), interleukin (IL)‐6 (F), and IL‐1β (G), whose expression were correlated with those of CSTA in sera.
Cancer Sci 108 (2017) 2122–2129
Funding Information
No funding declared.
References
- 1. Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol 2009; 6: 699–708. [DOI] [PubMed] [Google Scholar]
- 2. Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an update. Dig Dis 2010; 28: 645–56. [DOI] [PubMed] [Google Scholar]
- 3. Strimpakos AS, Syrigos KN, Saif MW. The molecular targets for the diagnosis and treatment of pancreatic cancer. Gut Liv 2010; 4: 433–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet 2011; 378: 607–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burczynski ME, Twine NC, Dukart G et al Transcriptional profiles in peripheral blood mononuclear cells prognostic of clinical outcomes in patients with advanced renal cell carcinoma. Clin Cancer Res 2005; 11: 1181–9. [PubMed] [Google Scholar]
- 6. Han M, Liew CT, Zhang HW et al Novel blood‐based, five‐gene biomarker set for the detection of colorectal cancer. Clin Cancer Res 2008; 14: 455–60. [DOI] [PubMed] [Google Scholar]
- 7. Showe MK, Vachani A, Kossenkov AV et al Gene expression profiles in peripheral blood mononuclear cells can distinguish patients with non‐small cell lung cancer from patients with nonmalignant lung disease. Cancer Res 2009; 69: 9202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mesko B, Poliska S, Szegedi A et al Peripheral blood gene expression patterns discriminate among chronic inflammatory diseases and healthy controls and identify novel targets. BMC Med Genomics 2010; 5: 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sakai Y, Honda M, Fujinaga H et al Common transcriptional signature of tumor‐infiltrating mononuclear inflammatory cells and peripheral blood mononuclear cells in hepatocellular carcinoma patients. Cancer Res 2008; 68: 10267–79. [DOI] [PubMed] [Google Scholar]
- 10. Komura T, Sakai Y, Honda M, Takamura T, Matsushima K, Kaneko S. CD14 + monocytes are vulnerable and functionally impaired under endoplasmic reticulum stress in patients with type 2 diabetes. Diabetes 2010; 59: 634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Komura T, Sakai Y, Harada K et al Inflammatory features of pancreatic cancer highlighted by monocytes/macrophages and CD4 + T cells with clinical impact. Cancer Sci 2015; 106: 672–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Honda M, Sakai Y, Yamashita T et al Differential gene expression profiling in blood from patients with digestive system cancers. Biochem Biophys Res Commun 2010; 400: 672–86. [DOI] [PubMed] [Google Scholar]
- 13. Verrecchia F, Mauviel A. TGF‐beta and TNF‐alpha: antagonistic cytokines controlling type I collagen gene expression. Cell Signal 2004; 16: 873–80. [DOI] [PubMed] [Google Scholar]
- 14. Barrett AJ. The cystatins: a diverse superfamily of cysteine peptidase inhibitors. Biomed Biochim Acta 1986; 45: 1363–74. [PubMed] [Google Scholar]
- 15. Jarvinen M, Rinne A, Hopsu‐Havu VK. Human cystatins in normal and diseased tissues: a review. Acta Histochem 1989; 82: 5–18. [DOI] [PubMed] [Google Scholar]
- 16. Sloane BF, Dunn JR, Honn KV. Lysosomal cathepsin B: correlation with metastatic potential. Science 1981; 212: 1151–3. [DOI] [PubMed] [Google Scholar]
- 17. Joyce JA, Baruch A, Chehade K et al Cathepsin cysteine proteases are effectors of invasive growth and angiogenesis during multistage tumorigenesis. Cancer Cell 2004; 5: 443–53. [DOI] [PubMed] [Google Scholar]
- 18. Akkari L, Gocheva V, Kester JC et al Distinct functions of macrophage‐derived and cancer cell‐derived cathepsin Z combine to promote tumor malignancy via interactions with the extracellular matrix. Genes Dev 2014; 28: 2134–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Olson OC, Joyce JA. Cysteine cathepsin proteases: regulators of cancer progression and therapeutic response. Nat Rev Cancer 2015; 12: 712–29. [DOI] [PubMed] [Google Scholar]
- 20. Ebert MP, Kruger S, Fogeron ML et al Overexpression of cathepsin B in gastric cancer identified by proteome analysis. Proteomics 2005; 5: 1693–704. [DOI] [PubMed] [Google Scholar]
- 21. Fernandez PL, Farre X, Nadal A et al Expression of cathepsins B and S in the progression of prostate carcinoma. Int J Cancer 2001; 95: 51–5. [DOI] [PubMed] [Google Scholar]
- 22. Kos J, Werle B, Lah T, Brunner N. Cysteine proteinases and their inhibitors in extracellular fluids: markers for diagnosis and prognosis in cancer. Int J Biol Markers 2000; 15: 84–9. [DOI] [PubMed] [Google Scholar]
- 23. Niedergethmann M, Wostbrock B, Sturm JW et al Prognostic impact of cysteine proteases cathepsin B and cathepsin L in pancreatic adenocarcinoma. Pancreas 2004; 29: 204–11. [DOI] [PubMed] [Google Scholar]
- 24. Ohta T, Terada T, Nagakawa T et al Pancreatic trypsinogen and cathepsin B in human pancreatic carcinomas and associated metastatic lesions. Br J Cancer 1994; 69: 152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gopinathan A, Denicola GM, Frese KK et al Cathepsin B promotes the progression of pancreatic ductal adenocarcinoma in mice. Gut 2012; 61: 877–84. [DOI] [PubMed] [Google Scholar]
- 26. Parker BS, Ciocca DR, Bidwell BN et al Primary tumour expression of the cysteine cathepsin inhibitor Stefin A inhibits distant metastasis in breast cancer. J Pathol 2008; 214: 337–46. [DOI] [PubMed] [Google Scholar]
- 27. Aničin A, Gale N, Smid L, Kos J, Strojan P. Expression of stefin A is of prognostic significance in squamous cell carcinoma of the head and neck. Eur Arch Otorhinolaryngol 2013; 270: 3143–51. [DOI] [PubMed] [Google Scholar]
- 28. Butler MW, Fukui T, Salit J et al Modulation of cystatin A expression in human airway epithelium related to genotype, smoking, COPD, and lung cancer. Cancer Res 2011; 71: 2572–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin YY, Chen ZW, Lin ZP et al Tissue levels of stefin A and stefin B in hepatocellular carcinoma. Anat Rec 2016; 299: 428–38. [DOI] [PubMed] [Google Scholar]
- 30. Bird PI, Trapani JA, Villadangos JA. Endolysosomal proteases and their inhibitors in immunity. Nat Rev Immunol 2009; 9: 871–82. [DOI] [PubMed] [Google Scholar]
- 31. Vray B, Hartmann S, Hoebeke J. Immunomodulatory properties of cystatins. Cell Mol Life Sci 2002; 59: 1503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zavasnik‐Bergant T. Cystatin protease inhibitors and immune functions. Front Biosci 2008; 1: 4625–37. [DOI] [PubMed] [Google Scholar]
- 33. Turk V, Stoka V, Turk D. Cystatins: biochemical and structural properties, and medical relevance. Front Biosci 2008; 13: 5406–20. [DOI] [PubMed] [Google Scholar]
- 34. Chunsun L, Liwei C, Jialing W et al Expression and clinical significance of cathepsin B and stefin A in laryngeal cancer. Oncol Rep 2011; 26: 869–75. [DOI] [PubMed] [Google Scholar]
- 35. Dennemarker J, Lohmüller T, Mayerle J et al Deficiency for the cysteine protease cathepsin L promotes tumor progression in mouse epidermis. Oncogene 2010; 29: 1611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park B, Brinkmann MM, Spooner E et al Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll‐like receptor 9. Nat Immunol 2008; 9: 1407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang J, He P, Zhong Q et al Increasing cystatin C and cathepsin B in serum of colorectal cancer patients. Clin Lab 2017; 63: 365–71. [DOI] [PubMed] [Google Scholar]
- 38. Monsouvanh A, Proungvitaya T, Limpaiboon T et al Serum cathepsin B to cystatin C ratio as a potential marker for the diagnosis of cholangiocarcinoma. Asian Pac J Cancer Prev 2014; 15: 9511–5. [DOI] [PubMed] [Google Scholar]
- 39. Yan Y, Zhou K, Wang L, Wang F, Chen X, Fan Q. Clinical significance of serum cathepsin B and cystatin C levels and their ratio in the prognosis of patients with esophageal cancer. Onco Targets Ther 2017; 10: 1947–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bruchard M, Mignot G, Derangère V et al Chemotherapy‐triggered cathepsin B release in myeloid‐derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med 2013; 19: 57–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Gene expression of CSTA, transcriptional factors, and cytokines in peripheral CD4+ T cells of patients with pancreatic ductal adenocarcinoma.
Fig. S2. Serum concentrations of cytokines and cystatin A (CSTA) in patients with pancreatic ductal adenocarcinoma (PDAC).
Fig. S3. Immunohistochemical analysis of with pancreatic ductal adenocarcinoma tissues for transcriptional factors and cytokines related to CD4+ T subsets T‐bet (A), Foxp3 (B), γ‐interferon (IFN‐γ) (C), and transforming growth factor‐β (TGF‐β) (D), as well as cytokines tumor necrosis factor‐α (TNF‐α) (E), interleukin (IL)‐6 (F), and IL‐1β (G), whose expression were correlated with those of CSTA in sera.


