Abstract
The proper culture conditions for producing cellulase of Bacillus amyloliquefaciens S1, isolated from the cecum of goose was optimized by single-factor experiment combined with orthogonal test. The properties of the cellulase were investigated by DNS method. The appropriate doses of B. amyloliquefaciens S1 were obtained by adding them to goose feed. It indicated that the suitable culture conditions of producing cellulase were the culture temperature of 37°C, the initial pH of 7.0, the incubation time of 72 h and the loaded liquid volume of 75 ml per 250 ml. The effects of each factor on producing cellulase by B. amyloliquefaciens S1 were as follows: initial pH > incubation time = culture temperature > loaded liquid volume. The optimum reaction temperature and pH were 50°C and 7.0, respectively. This enzyme is a kind of neutral cellulase that possesses resistance to heat and acidity. It showed high activity to absorbent cotton, soya bean meal and filter paper. By adding different doses of B. amyloliquefaciens S1 to the goose feed, it was found that the egg production, average egg weight, fertilization rate and the hatching rate were promoted both in experiment 1 (1.5 g kg−1) and experiment 2 (3 g kg−1). Also the difference of egg production, fertilization rate and hatching rate between experiment 1 and control group was obvious (p < 0.05), and the average egg weight was significantly increased in experiment 2 (p < 0.05).
Keywords: Bacillus amyloliquefaciens S1, cecum of goose, cellulase, culture conditions, enzyme properties, goose feed
1. Introduction
Cellulose is one of the most abundant biodegradable materials on the Earth [1] which can be produced by many organisms, including bacteria and vascular plants [2–4]. Cellulase is an enzymatic complex composed of endo-1,4-β-d-glucanases or endoglucanases, exo-1,4-β-d-glucanases or cellobiohydrolases and 1,4-β-d-glucosidases, which act on cellulose to produce glucose [5,6]. As a kind of important industrial enzyme, cellulase has been widely used in the feed industry, alcoholic fermentation, fruit juice and other fields [7,8]. The utilization of cellulase in animal feed has been reported widely [9,10].
Probiotics offer a promising alternative to chemicals and antibiotics in animal feed [11]. They can increase beneficial gut commensal bacteria which are beneficial to the host's digestion, enhancing growth and immune responses, and inhibiting pathogenic microorganisms [12–14]. So the cellulase and probiotics can be applied in the feed industry to improve gut health of animal and digestibility of the feed. Bacillus is a kind of probiotics that can secrete high activity of protease, lipase, amylase and cellulase. Bacillus amyloliquefaciens is an important potential probiotic strain [15,16] that has been found to secrete cellulase [17] and applied to many types of mammalian feed for improving their intestinal microenvironment.
Goose is a type of feeding forage-saving plant-eating waterfowl. As herbivorous poultry, geese like eating grass, green vegetables and other plants rich in crude fibre [18]. Geese have a very strong ability to digest dietary fibre [19–21]. In contrast to chickens and ducks, geese have a maximum requirement for dietary crude fibre. After being fed with very low crude fibre content, geese experience a decline in growth rate and an increase in mortality. The muscular stomach of the goose is mainly used to grind food, promote digestion and the proventriculus stomach secretes digestive enzymes and minerals to digest food. A pair of well-developed cecum of goose can use a lot of crude fibre. There is a lot of cellulose contained in the cecum of goose [19]. While the cecum of goose cannot secrete digestive enzyme, the cellulase mainly comes from the microorganisms in the cecum [22]. So the cecum of goose has a very high activity of cellulase to digest cellulose. Some ruminants can use large quantities of low-quality roughages as energy sources by microbial degradation of fibre in the gastrointestinal tract [23]. However, most other animals do not have this ability to use the cellulose [24]. As the cecum of goose has the same fermentation function as the ruminant rumen, goose can use a lot of crude fibre. But the effect of cellulase used in goose feed can be affected by many factors, such as the health conditions, age of geese, composition of the feed, composition and quantity of the microorganisms in the cecum, and the physiological differences between different individuals. Cellulolytic bacteria make a great contribution to the energy supply for foraging animals. Feed fibres cannot be completely used by animals and 20–70% of the cellulose is carried out with faeces [25]. Therefore, it is possible to combine the probiotic attributes of a Bacillus strain and its cellulose degrading capability to enhance the digestibility of animal feed and the productivity of animals.
Increasing concerns regarding antibiotic resistance and the presence of drug residues in animal products have led several European countries and South Korea to ban the use of antibiotics in animal feed [26,27]. However, it is feared that the ban of antibiotics may have adverse consequences for animal health and farmers' profits. This has triggered a search for viable alternatives to antibiotics in the animal industry. Probiotics have serious potential for this application [28]. Promising results have been found upon the application of probiotics in the poultry industry [29]. The supplementation of various probiotics has been shown to diversify and stabilize gastrointestinal microbiota [30], in addition to improving animal production and health [31]. However, the effectiveness of probiotics in animal studies varies greatly depending on the origin of the microbes [32]. Lactobacillus species, yeast species and spore-forming bacteria such as Bacillus species are the species used as probiotics [33]. Although B. amyloliquefaciens is a member of genus Bacillus, limited studies have been conducted to assess its efficacy in goose feed.
In the present experiment, the optimization of fermentation conditions and properties of the cellulase of one cellulase-producing bacterium isolated from the cecum of goose will be investigated. This experiment provides a reliable theoretical basis for the application of cellulolytic bacteria, which probably solves the difficulties of practical application in reality. In addition, Wanxi white geese were used as research animal in this experiment. The effects of adding 1.5 and 3.0 g kg−1 of B. amyloliquefaciens feed additive on the egg production, average egg weight, egg fertilization rate and hatching rate of Wanxi white geese were studied. It provides further theoretical basis for the high efficiency production and green production of geese products.
2. Material and methods
2.1. Material and reagents
Bacillus amyloliquefaciens S1 isolated from the cecum of goose provided by Laboratory of Physiology and Biochemistry, Anhui Agricultural University [34].
LB culture consists of tryptone 1.0%, yeast extract 0.5%, NaCl 1.0% and pH 7.0.
Fermentation medium consists of bran 2.0%, soya bean meal 3.0%, CMC 0.5% and NaCl 0.5%.
Ninety Wanxi white geese with similar weight and good health were chosen from Luan Zhanyu Company, including 18 male geese and 72 female geese.
2.2. Methods
2.2.1. Preparation of crude enzymes
The cellulase secretion strain was inoculated into the fermentation medium. The fermentation broth was continuously shaken at 37°C for 48 h at 200 r.p.m. to produce cellulase. After cultivation, the cultured liquid mixture of the bacteria was centrifuged at 6000 r.p.m. for 10 min at 4°C by high-speed freezing centrifuge to obtain a crude enzyme and the liquid was maintained at 4°C. The activity of cellulase was determined by DNS method.
2.2.2. Optimization of culture conditions
Optimization of temperature. To determine the effective temperature for cellulase production by the bacterial strains, fermentation was carried out at 21°C, 29°C, 37°C, 45°C and 53°C.
Optimization of incubation time. Some microorganisms produce maximally during their exponential phase, whereas others in their stationary growth phase. The fermentation was carried out from 24 to 108 h, the production rate was measured at 12 h intervals.
Optimization of initial pH. The most suitable pH of the fermentation medium was determined by adjusting the pH of the culture medium to 5.0, 6.0, 7.0, 8.0 and 9.0.
Optimization of liquid load. To test the effect of different liquid load on cellulase production by the strains, 250 ml Erlenmeyer flasks were filled with different volumes of fermentation broth (50, 75, 100, 125, 150 ml).
Optimization of carbon and nitrogen ratio. The optimal proportion of carbon and nitrogen sources for the production of enzymes was determined by changing the added proportions to 1 : 9, 1 : 4, 2 : 3, 1 : 1, 3 : 2, 4 : 1 and 9 : 1.
2.2.3. Orthogonal test
An L9 (34) orthogonal table was chosen using the cellulose activity value of the fermentation supernatant fluid as the inspection index, and cultivation temperature (A), incubation time (B), pH value (C) and liquid load (D) were used as the experimental factors. Each factor was designed with three experiment levels, the factors and levels of orthogonal tests for fermentation are shown in table 1.
Table 1.
Factors and levels of orthogonal tests for fermentation.
| level | A (cultivate temperature (°C)) | B (incubation time (h)) | C (pH value) | D (liquid load (ml)) |
|---|---|---|---|---|
| 1 | 29 | 60 | 5 | 50 |
| 2 | 37 | 72 | 6 | 75 |
| 3 | 45 | 84 | 7 | 100 |
2.2.4. Properties of the cellulase
Optimal temperature of enzyme reaction. The optimum temperature of the enzyme was determined by performing the assay in the range of 30–75°C with an interval of 5°C. And the relative activity was calculated with respect to maximum exhibited activity of 100%.
Optimal pH of enzyme reaction. The effect of different pH (3.0, 5.0, 6.0, 7.0 and 8.0) on the activity of cellulase was evaluated at suitable temperatures.
Thermal stability of the enzymes. To test the thermal stability, the enzyme was measured by incubating it in a water bath at 50°C, 55°C, 60°C, 65°C, 70°C and 75°C for 10, 20, 30, 60, 120 and 240 min, respectively. The residual activity was recorded as previously described.
pH stability of the enzyme. To investigate the pH stability, the enzyme was incubated at different pH values for 17 h at 30°C. The residual activity of each sample was measured as described above.
Effect of various metal ions. The effect of various known metal ions such as K+, Cu2+, Fe2+, Fe3+, Mn2+ and Zn2+ on the cellulase was studied at a 1 mmol l−1 concentration. Control, without metal ion, was maintained. The relative activity was measured with respect to the control group where the reaction was carried out in the absence of any metal ions under the optimum assay conditions.
Selectivity of enzyme to substrates. Untreated CMC-Na (control group), cassava dregs, absorbent cotton, soya bean meal, filter paper and microcrystalline cellulose were used as the substrate. The cellulase activity was measured under the optimum assay conditions.
2.2.5. Feeding experiment
Animals and grouping. Ninety Wanxi white geese with similar weight and good health were chosen for the feeding experiment. Geese were house in sterile pens. Ninety geese were randomized into three groups (control group, experiment group 1 and experiment group 2) equally based on body weight and external characteristics, and six repeat sets, each include five geese (one male and four female). Geese of the control group were fed on the original basic diet without adding any additional ingredients. Geese of the experiment group 1 were fed on basic diet supplemented with B. amyloliquefaciens 1.5 g per 1 kg, and the experiment group 2 was fed on the basic diet with the addition of B. amyloliquefaciens 3.0 g per 1 kg. The basic diet is the base feed which is made of original factory corn flour and rice in equal quantities.
Feeding management. The geese house was disinfected and segregated before the start of the experiment and breeding geese by means of flat farming. During the trial period, the geese were free to move, feed and drink water; other methods were kept constant in the daily habit of the geese. We kept daily light time and conducted epidemic prevention and disinfection measures according to routine procedures. The health condition, feeding, drinking, movements and disease rate of the geese were observed every day during the feeding period.
Data acquisition and analysis. The geese were fed quantitatively once a day, and the eggs collected once a day. The data of egg production, egg weight and feed consumption were recorded, and the numbers of repeats on the geese were marked for recording. Each group was divided into 10 groups by feed consumption of 100 kg a week. When the egg production reached a certain number, the eggs were sent to the incubation room to hatch uniformly. Incubation period is usually 30 days; the egg fertilization rate and incubation rate were recorded during hatching period. All data were analysed by Excel and then statistical analysis was analysed by one-way ANOVA procedures of SPSS 22.1. All the values were considered significant at p < 0.05 and were expressed as mean ± s.e.
3. Results
3.1. Optimization of culture conditions
3.1.1. Effect of culture temperature on enzyme production
The strains were cultured in 100 ml of fermentation medium for 48 h at 21°C, 29°C, 37°C, 45°C and 53°C. The strain had the strongest ability to produce cellulase with the fermentation temperature at 37°C (figure 1a). With increasing temperature, the activity of enzymes first increased and then decreased. Temperature either below or above 37°C was not optimal for yielding the enzymes. Therefore, the cultivating temperature was set at 37°C in the following tests.
Figure 1.
Optimization of fermentation conditions of cellulase secreted by B. amyloliquefaciens S1. (a) Effect of fermentation temperature on enzyme production; (b) effect of fermentation time on enzyme production; (c) effect of initial pH of medium on enzyme production; (d) effect of carbon and nitrogen ratio of medium on enzyme production and (e) effect of medium volume aeration on enzyme production.
3.1.2. Effect of fermentation time on enzyme production
The bacterium was cultivated at the optimal temperature (37°C), and the enzyme activity was measured at 12 h intervals. The activity of cellulase increased with the prolongation of culture time within 72 h and reached the maximum activity at 72 h; the activity of cellulase decreased significantly after 84 h (figure 1b).
3.1.3. Effect of initial pH of medium on enzyme production
Keeping the other conditions unchanged, the enzyme activity was measured at different initial pH (5.0, 6.0, 7.0, 8.0 and 9.0). The optimum initial pH value of the fermentation medium was 6.0, and the amount of enzyme was stable in the range of pH 5–6, while the ability of producing enzyme was decreased significantly at the initial pH 7–9 (figure 1c).
3.1.4. Effect of liquid load of medium on enzyme production
The volume of liquid in the flask was changed to study the effect of liquid load. The enzyme activity was measured at 37°C, pH 6 after cultivating for 72 h. As is shown in figure 1d, the optimal liquid load was 75 ml and more or less volume aeration suppresses the enzyme production.
3.1.5. Effect of carbon and nitrogen ratio of medium on enzyme production
Carbon and nitrogen sources are nutrients for bacteria growth; they are important for the growth and reproduction of bacteria. The incubation temperature was set to 37°C, shake culture time to 72 h, pH to 6.0, volume to 75 ml, and carbon and nitrogen ratio to 1 : 9, 1 : 4, 2 : 3, 1 : 1, 3 : 2, 4 : 1 and 9 : 1. The results (figure 1e) reveal that the strain showed better growth and higher enzymatic activity in the fermentation medium with carbon and nitrogen ratio of 1 : 1. When carbon–nitrogen ratio is more than 1 : 1, the enzyme production is stable, and the ability to produce cellulase increased gradually as the ratio increased at the carbon and nitrogen ratio less than 1 : 1.
3.1.6. The result of the orthogonal test
It can be seen from the R-value in the orthogonal table that bacterial enzyme production was affected most by pH value, followed by incubation temperature and time, and less so by medium volume. The best bacterial culture conditions are A2B2C2D2 according to their responsibility, respectively, which stands for an incubation temperature of 37°C, incubation time of 72 h, initial pH of 6.0, and medium volume of 75/250 ml (table 2).
Table 2.
Results of L9 (34) orthogonal test.
| treatment | A | B | C | D | enzyme activity (U ml−1) |
|---|---|---|---|---|---|
| 1 | A1 | B1 | C1 | D1 | 1.21 |
| 2 | A1 | B2 | C2 | D2 | 1.37 |
| 3 | A1 | B3 | C3 | D3 | 0.97 |
| 4 | A2 | B1 | C2 | D3 | 1.41 |
| 5 | A2 | B2 | C3 | D1 | 1.13 |
| 6 | A2 | B3 | C1 | D2 | 1.22 |
| 7 | A3 | B1 | C3 | D2 | 1.04 |
| 8 | A3 | B2 | C1 | D3 | 1.20 |
| 9 | A3 | B3 | C2 | D1 | 1.14 |
| K1 | 3.55 | 3.66 | 3.63 | 3.49 | |
| K2 | 3.76 | 3.70 | 3.91 | 3.63 | |
| K3 | 3.38 | 3.33 | 3.15 | 3.58 | |
| k1 | 1.18 | 1.22 | 1.21 | 1.16 | |
| k2 | 1.25 | 1.23 | 1.30 | 1.21 | |
| k3 | 1.13 | 1.11 | 1.05 | 1.19 | |
| R | 0.12 | 0.12 | 0.25 | 0.05 |
3.2. Enzymatic properties
3.2.1. Optimum enzyme reaction temperature and thermal stability of cellulase
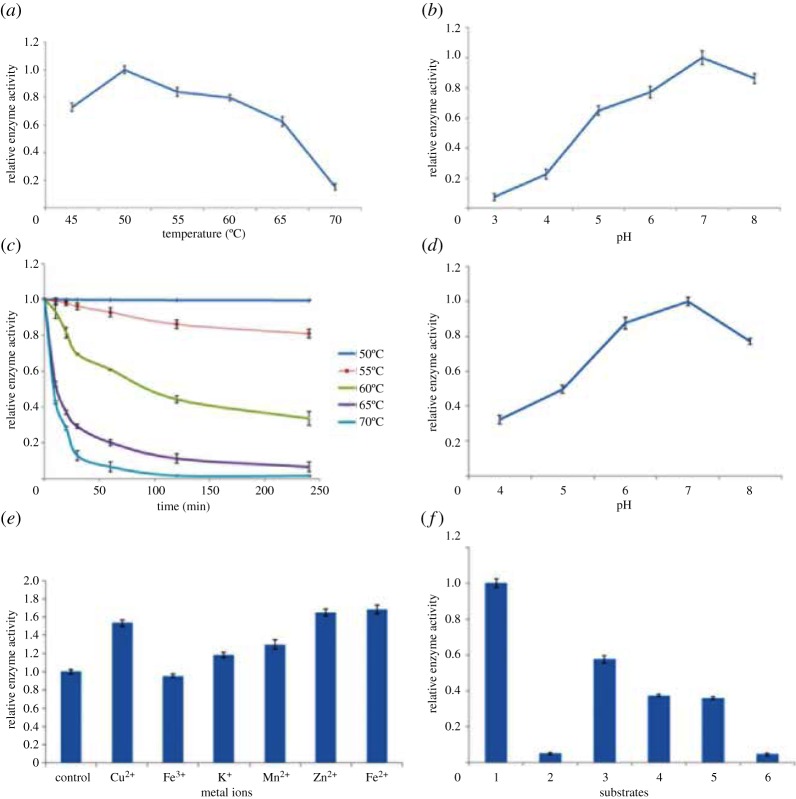
Cellulase activity at various temperatures was measured using CMC (carboxymethylcellulose salt) as a substrate. The results (figure 2a) showed that the appropriate temperature of the cellulase reaction ranged from 45°C to 55°C, and the optimum temperature was 50°C. More than 97% of cellulase activity was retained even upto 4 h at 50°C; at the temperature of 55°C, the enzyme activity remained at 77% after 4 h. Moreover, cellulase activity was reduced by 77% at 60°C after 4 h. Whereas, the cellulase activity decreased drastically at 65°C after 1 h and 70°C after 30 min (figure 2c).
Figure 2.
Properties of cellulase secreted by B. amyloliquefaciens S1. (a) Effect of temperature on cellulase activity; (b) effect of pH on cellulase activity; (c) thermal stability; (d) pH stability; (e) effect of metal ions on cellulase activity and (f) selectivity of cellulase to substrates. 1: CMC-Na; 2: cassava dregs; 3: absorbent cotton; 4: soya bean meal; 5: filter paper; 6: microcrystalline cellulose.
3.2.2. Optimum enzyme reaction pH and pH stability of the cellulase
The activity of the cellulase fermented at different pH (4.0, 5.0, 6.0, 7.0 and 8.0) was measured at 50°C. As is shown in figure 2b, the optimum pH of enzymatic reaction was 7.0. Cellulase activity was reduced 23% at pH 6 and 14% at pH 8. In the aspect of pH stability, the enzyme activity was stable at the range 5.0–7.0, but the enzyme activity is not high, it may be affected by pH. The enzyme activity reached the highest at pH 7.0, and the enzyme activity decreases rapidly when the pH is above 7.0. The enzyme activity was reduced 12.4% at pH 6.0, but decreased 23.0% at pH 8.0 (figure 2d). This result indicates that cellulase from B. amyloliquefaciens S1 can be used in neutral to slightly acidic environments.
3.2.3. Effect of metal ions on cellulase activity
Metal ions play a major role as cofactors in enzymatic activity. The presence of Cu2+, K+, Mn2+, Zn2+ and Fe2+ would enhance the activity of cellulase by 53.4%, 18.0%, 29.9%, 64.9% and 68.5%, respectively. The presence of Fe3+ at 1 mmol l−1 produced a slight effect on the cellulase by reducing the activity to 94.89% of its initial activity (figure 2e).
3.2.4. The selectivity of cellulase to substrates
The decomposition capacities of cellulase to the substrates were different. The cellulase showed a greater activity with absorbent cotton (57.5%), soya bean meal (37.7%) and filter paper (36.2%) compared with that of the control. However, the activities with cassava dregs (5.0%) and microcrystalline cellulose (3.6%) were weak (figure 2f).
3.3. Bacillus amyloliquefaciens supplemented in feed for Wanxi white geese
3.3.1. Effects of Bacillus amyloliquefaciens on egg production and average egg weight
The addition of B. amyloliquefaciens to the geese feed not only had a certain effect on improving the fertilization rate, but also improved the hatching rate of geese eggs to a certain extent (table 3). Compared with the control group, the number of eggs produced in the experimental group 1 increased by 21.14% (p = 0.0140 < 0.05) and the average egg weight increased by 3.22% (p = 0.1770 > 0.05). And the total number of eggs increased by 8.37% (p = 0.0079 < 0.05), and the average egg weight increased by 6.17% (p = 0.0300 < 0.05) in experiment group 2. The number of eggs laid in both groups was improved, compared with the control group, but only the experimental group 1 was significantly improved; the average egg weight had a tendency to increase, but only the experimental group 2 was significantly improved (p < 0.05), the difference may be caused by the ratio of addition of B. amyloliquefaciens S1.
Table 3.
Effect of B. amyloliquefaciens on egg production and average egg weight. One means control group; two means experiment group 1; three means experiment group 2.
| number of eggs |
average egg weight (g) |
|||||
|---|---|---|---|---|---|---|
| weeks | one | two | three | one | two | three |
| 1–2 weeks | 91 | 101 | 96 | 172.73 ± 5.66 | 179.80 ± 4.65 | 185.80 ± 3.79 |
| 3–4 weeks | 63 | 69 | 70 | 171.00 ± 7.18 | 172.00 ± 10.82 | 183.10 ± 5.97 |
| 5–6 weeks | 36 | 35 | 27 | 164.20 ± 10.44 | 166.60 ± 6.76 | 179.90 ± 6.94 |
| 7–8 weeks | 35 | 53 | 34 | 157.10 ± 7.15 | 156.00 ± 6.08 | 180.80 ± 12.08 |
| 9–10 weeks | 12 | 71 | 19 | 150.40 ± 20.01 | 167.30 ± 8.82 | 178.10 ± 35.03 |
| total/average | 227a | 275b | 246b | 163.08 ± 9.39a | 168.32 ± 8.65a | 180.56 ± 3.96b |
a,b Different lower case superscript were significantly different by the one-way ANOVA means in a row (p < 0.05). Data are reported as mean ± s.e.
3.3.2. Effect of Bacillus amyloliquefaciens on fertilization rate and hatching rate
The addition of B. amyloliquefaciens to the feed of geese has a certain effect on improving the fertilization rate, and the hatching rate of geese eggs also was improved to a certain extent (table 4). Compared with the control group, the fertilization rate in the experimental group 1 increased by 13.97% (p = 0.024 < 0.05), and the hatching rate increased by 11.89% (p = 0.023 < 0.05); besides, the fertilization rate increased by 7.25% (p = 0.153 > 0.05), and the hatching rate increased by 6.58% (p = 0.190 > 0.05) in experiment group 2. It can be seen that the addition of different proportions of B. amyloliquefaciens made the egg fertilization rate and hatching rate improve to varying degrees (the fertilization rate and hatching rate in experimental group 1 was significantly higher than that in experimental group 2).
Table 4.
Effect of B. amyloliquefaciens on fertilization rate and hatching rate. One means control group; two means experiment group 1; three means experiment group 2.
| fertilization rate (%) |
hatching rate (%) |
|||||
|---|---|---|---|---|---|---|
| days | one | two | three | one | two | three |
| 1 | 82.81 ± 5.47 | 85.00 ± 4.75 | 83.67 ± 7.97 | 84.91 ± 14.90 | 85.00 ± 4.50 | 83.67 ± 7.17 |
| 2 | 79.10 ± 9.29 | 92.19 ± 4.42 | 96.55 ± 4.63 | 79.17 ± 10.96 | 92.19 ± 7.94 | 96.56 ± 4.69 |
| 3 | 61.54 ± 16.12 | 90.00 ± 10.54 | 84.38 ± 8.51 | 72.22 ± 28,42 | 90.00 ± 9.13 | 84.38 ± 11.95 |
| 4 | 80.00 ± 40.05 | 92.86 ± 21.71 | 80.00 ± 14.96 | 80.00 ± 51.64 | 92.86 ± 6.81 | 80.00 ± 54.77 |
| 5 | 84.00 ± 5.12 | 87.50 ± 7.08 | 90.00 ± 14.11 | 84.00 ± 19.61 | 87.50 ± 13.54 | 90.00 ± 16.05 |
| 6 | 75.00 ± 14.47 | 100.00 ± 14.91 | 71.43 ± 25.82 | 75.00 ± 54.77 | 100.00 ± 51.63 | 71.43 ± 51.64 |
| average | 77.09 ± 8.23a | 91.26 ± 5.19b | 84.34 ± 8.57a | 79.22 ± 4.95a | 91.11 ± 5.09b | 85.80 ± 8.44b |
a,bDifferent lower case superscript were significantly different by the one-way ANOVA means in a row (p < 0.05). Data are reported as mean ± s.e.
4. Discussion
4.1. Optimization of culture conditions
As a major component of plants, cellulose accounts for almost half of the plant dry weight [35]. Therefore, there is a certain amount of cellulose contained in animal feed which reduces the digestibility of feed for most animals. Ruminant rumen contains a variety of microorganisms that can secrete cellulase, so these microorganisms can hydrolyse the cellulose in the feed; as a result, the digestion and utilization of nutrients were improved greatly. Cellulase-producing microorganisms are widely studied [36]. Current research has shown that it was an ideal method to decompose cellulose by using cellulase to degrade cellulose. The decomposition of cellulose can not only lead to the natural cellulose resources being fully used, but also reduce the anti-nutritional effect of crude fibre in feed [37].
There are many kinds of optimization method, such as response surface method [38], single-factor method and so on. In this experiment, single-factor and orthogonal test method was used to optimize the fermentation conditions. The study of the fermentation conditions of cellulase was of great significance to the production and application of cellulase. The optimum fermentation temperature for cellulase of the B. amyloliquefaciens S1 is similar to some other Bacillus sp. [39–41]. The optimum fermentation time of B. amyloliquefaciens S1 was 72 h, which was much shorter than most researches reported. For example, the optimum fermentation conditions of Bacillus SSP-34 showed that the cellulase activity reached the highest at 96 h [42]. Besides that, the optimal fermentation time of cellulase for B. amyloliquefaciens was 48 h, which had also been reported [43]. The cell growth and the fermentation of the strain were greatly affected by the initial pH of the fermentation medium. The growth of bacterium was under the influence of extreme pH conditions. In addition, the optimum initial pH was 6.0, which is similar to that of Kohli et al. [44]. Besides, some researches have shown that the optimal fermentation pH of B. pumilus ASH [39], B. circulans AB 16 [45], B. subtilis ASH [42] and B. qingshengii sp. nov. [46] was at pH 7.0. The production of enzyme is stable at optimum ratio of carbon and nitrogen of more than 1 : 1, which is consistent with the theory that carbon source is essential for the growth of microorganisms. The liquid volume mainly affects the capacity of the fermentation oxygen.
4.2. Enzymatic properties
In order to use the cellulase effectively, the experiment also studied the enzymatic properties of cellulase. The optimal temperature and pH for the crude enzyme was 50°C and 7.0, respectively. It was different from the results of Sun et al. [34]. Because Sun et al. added ammonium sulfate solids to 70% saturation in the crude enzyme solution, this processing resulted in some enzymatic properties changes of the crude enzyme after primary purification. The cellulase had a good stability. The remaining enzyme activity was about 25% after maintaining at 60°C for 4 h. But the remaining enzyme activity is almost 0 after incubating at 65 and 70°C for an hour. The stable pH range of the enzyme in this experiment is 6.0–7.0 which belongs to neutral enzyme and poor alkali-resistance, the same as some studies on alkali-resistance and acid-resistance cellulase in recent years [47–50]. The strain is suitable for exogenous feed enzymes owing to its character as suitable in the animal digestive tract environment. Except Fe3+, which has a small inhibitory effect on cellulase activity, the other metal ions have a certain role in promoting enzyme activity, which means that the role of the enzyme depends on the activation of metal ions. The cellulase secreted by the strain had strong ability to decompose the absorbent cotton, soya bean meal and filter paper, which indicated that the specificity of the enzyme was suitable for decomposing the fibre in animal feed.
4.3. Bacillus amyloliquefaciens supplemented in feed for Wanxi white goose
Spore-forming Bacillus spp. have been used as probiotics for their beneficial qualities to human and animal health [28]. A large number of Bacillus-based preparations have been found to promote growth, feed utilization and digestive health, subsequently, registered as probiotics for animal feed [51–53]. Egg production performance determines the economic benefits in laying hen production system owing to its effects on productivity [54]. Similar to our study, geese fed with 109 cfu g−1 Bacillus subtilis can increase growth performance and leg muscle weight (p < 0.05) because of its modulating the intestinal microflora ecology of the animal [55]. It was proved that the usage of 250 mg kg−1 B. subtilis culture in the diet significantly improves the body weight and feed consumption of goslings [56]. Hens fed with 2 × 106 cfu g−1 and 1.2 × 107 cfu g−1 B. licheniformis had higher egg production than those fed diet without the organism, while hens fed with diets containing 4 × 106 cfu g−1, 6 × 106 cfu g−1 and 1.8 × 107 cfu g−1 B. licheniformis had intermediate egg production (p < 0.05) [57]. Abdelqader et al. [58] reported that hens fed with diet supplemented with 2.3 × 108 cfu g−1 B. subtilis PB6 had higher egg production and feed conversion than the above. In this study, the number of eggs laid in experiment groups 1 and 2 were significantly higher than that in the control group (21.14% and 8.37%), but the difference was not significant. The analysis may be due to the fact that animal intestinal microbial flora and the microenvironment had already reached their relative balance. But the effect is not obvious and not necessarily reflected in the production performance, although Bacillus still has the effect on improving feed conversion. Scheuermann et al. [59] also argued that the viability of preparations for animal intestinal microbial balance did not necessarily reflect the performance through the production.
Bacillus can change the acid–base environment, secrete various enzymes, promote the absorption of various nutrients and maintain the balance of the microorganisms by secreting antimicrobial substances and acidic substances, which can be applied to enhancing the egg quality of poultry. Respective microbial feed additives can enrich for specific bacterial community members and modulate the diversity of the microbiome influencing microbiome composition in a predictable way. But diet with microbial feed additives may have indirect effects on weight gain and feed conversion through the microbiome [60]. Microbial feed additives beneficial to animal metabolism, the various enzymes and other unknown factors can stimulate the reproductive system of poultry, enhance sperm, egg number and quality, thereby enhance the animal fertility rate. In this experiment, the fertilization rate and hatching rate of Wanxi white geese eggs were increased with the addition of B. amyloliquefaciens. However, through the significance analysis, there were significant differences between experiment 1 and experiment 2 and the effect of experiment 1 (1.5 g kg−1), comparing with that of experiment 2, was significant improved (p < 0.05), but the effect difference by the addition with B. amyloliquefaciens in the experimental group 2 (3.0 g kg−1) was not significant (p > 0.05). In this experiment, the fertilization rate and hatching rate of geese were significantly higher than those of the control group, while the experimental group 2 was not significantly higher than the control. Lei et al. [61] used basal diet supplemented with different ratios of B. amyloliquefaciens and indicated that different ratios of B. amyloliquegaciens have a different effect on chicken growth performance. It was calculated, in a certain scale, that the less addition of B. amyloliquefaciens in poultry, the better the experimental effect of the experimental group. The optimal dose of B. amyloliquefaciens applied to goose feed should be further studied in future.
5. Conclusion
The optimal enzyme-producing conditions of B. amyloliquefaciens were culture temperature 37°C, incubation time 72 h, pH 6.0, outfit fluid amount 75 ml per 250 ml, and carbon to nitrogen ratio of 1 : 1. The properties of the cellulase indicated that the best pH for the activity of the enzymes was 7.0 and the optimum reaction temperature was 50°C. The enzyme was neutral cellulase, possessing resistance to heat and acidity.
B. amyloliquefaciens (1.5 g kg−1 and 3.0 g kg−1) was added to the feed of the Wanxi white goose. The number of eggs produced and the average egg weight were increased (p > 0.05) in the experiment 2 (1.5 g kg−1), while the effect of adding 3.0 g kg−1 B. amyloliquefaciens to feed on the average egg weight of geese was significant (p < 0.05). Moreover, both adding 1.5 g kg−1 and 3.0 g kg−1 of B. amyloliquefaciens to the feed had a tendency to increase the fertilization rate and hatching rate of geese. Furthermore, the fertilization rate and hatching rate of goose eggs were significantly improved (p < 0.05). According to the comprehensive experimental data, the addition of B. amyloliquefaciens to the feed for Wanxi white goose could increase the production performance of the geese and help to improve the breeding income.
Supplementary Material
Data accessibility
The data supporting this article are available on Dryad: (https://doi.org/10.5061/dryad.27j11) [62].
Authors' contributions
Z.W. designed, performed and wrote the experiments, and helped draft the manuscript. M.Y .and L.S. carried out the laboratory work, participated in data analysis, participated in the design of the study and drafted the manuscript. R.Y. collected field data. All authors gave final approval for publication. K.Q. revised the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the Major Science and Technology Program of Anhui Province (2017), Anhui Industry and Technology System of Poultry Science (AHCYTX-10), Management and Demonstration of Quality and Safety Control Technology of Green Ecology Livestock and Poultry Products Industry Chain (1604A0702033), the Biology Key Subject Construction of Anhui, the FSKLAN (Fund of State Key Laboratory of Animal Nutrition: 2004DA125184F1418).
References
- 1.Mohite BV, Patil SV. 2014. A novel biomaterial: bacterial cellulose and its new era applications. Biotechnol. Appl. Biochem. 61, 101–110. (doi:10.1002/bab.1148) [DOI] [PubMed] [Google Scholar]
- 2.Romling U. 2002. Molecular biology of cellulose production in bacteria. Res. Microbiol. 153, 205–212. (doi:10.1016/S0923-2508(02)01316-5) [DOI] [PubMed] [Google Scholar]
- 3.Keegstra K. 2010. Plant cell walls. Plant. Physiol. 154, 483–486. (doi:10.1104/pp.110.161240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimura S, Ohshima C, Hirose E, Nishikawa J, Itoh T. 2001. Cellulose in the house of the appendicularian Oikopleura rufescens. Protoplasma. 216, 71–74. (doi:10.1007/BF02680133) [DOI] [PubMed] [Google Scholar]
- 5.Cai YJ, Chapman SJ, Buswell JA, Chang ST. 1999. Production and distribution of endoglucanase, cellobiohydrolase, and beta-glucosidase components of the cellulolytic system of Volvariella volvacea, the edible straw mushroom. Appl. Environ. Microbiol. 65, 553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhat MK, Bhat S. 1997. Cellulose degrading enzymes and their potential industrial applications. Biotechnol. Adv. 15, 583–620. (doi:10.1016/S0734-9750(97)00006-2) [DOI] [PubMed] [Google Scholar]
- 7.Ogel ZB, Yarangumeli K, Du H, Ifrij I. 2001. Submerged cultivation of Scytalidium thermophilum on complex lignocellulosic biomass for endoglucanase production. Enz. Microb. Technol. 28, 689–695. (doi:10.1016/S0141-0229(01)00315-5) [DOI] [PubMed] [Google Scholar]
- 8.Caargo D, Gomes SD, Sene L. 2014. Ethanol production from sunflower meal biomass by simultaneous saccharification and fermentation (SSF) with Kluyveromyces marxianus ATCC 36907. Bioprocess. Biosyst. Eng. 37, 2235–2242. (doi:10.1007/s00449-014-1201-x) [DOI] [PubMed] [Google Scholar]
- 9.Ribeiro T, et al. 2008. A family 11 carbohydrate-binding module (CBM) improves the efficacy of a recombinant cellulase used to supplement barley-based diets for broilers at lower dosage rates. Br. Poult. Sci. 49, 600–608. (doi:10.1080/00071660802345749) [DOI] [PubMed] [Google Scholar]
- 10.Rodhe AV, Sateesh L, Sridevi J, Venkateswarlu B, Venkateswar RL. 2011. Enzymatic hydrolysis of sorghum straw using native cellulase produced by T. reesei NCIM 992 under solid state fermentation using rice straw. Biotech. 1, 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khaksar V, Veldkamp T, Hashemipour H. 2014. Effect of a prebiotic on performance of partridge. J. Anim. Physiol. Anim. Nutr. 98, 511–516. (doi:10.1111/jpn.12100) [DOI] [PubMed] [Google Scholar]
- 12.Song SK, Beck BR, Kim D, Park J, Kim J, Kim HD, Ringø E. 2014. Prebiotics as immunostimulants in aquaculture: a review. Fish. Shellfish Immunol. 40, 40–48. (doi:10.1016/j.fsi.2014.06.016) [DOI] [PubMed] [Google Scholar]
- 13.Kos B, Suskovic J, Vukovic S, Simpraga M, Frece J, Matosic S. 2003. Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J. App. Microbiol. 94, 981–987. (doi:10.1046/j.1365-2672.2003.01915.x) [DOI] [PubMed] [Google Scholar]
- 14.Verschuere L, Rombaut G, Sorgeloos P, Verstraete W. 2000. Probiotic bacteria as biological control agents in aquaculture. Microbiol. Mol. Biol. Rev. 64, 655–671. (doi:10.1128/MMBR.64.4.655-671.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das A, Nakhro K, Chowdhury S, Kamilya D. 2013. Effects of potential probiotic Bacillus amyloliquifaciens FPTB16 on systemic and cutaneous mucosal immune responses and disease resistance of catla (Catla catla). Fish. Shellfish Immunol. 35, 1547–1553. (doi:10.1016/j.fsi.2013.08.022) [DOI] [PubMed] [Google Scholar]
- 16.Avdeeva LV, Nechypurenko OO, Kharhota MA. 2015. Probiotic features of carotene producing strains Bacillus sp. 1.1 and B. amyloliquefaciens UCM B-5113. Mikrobiol. Z. 77, 22–27. [PubMed] [Google Scholar]
- 17.Lee YJ, Kim BK, Lee BH, Jo KI, Lee NK, Chung CH. 2008. Purification and characterization of cellulase produced by Bacillus amyoliquefaciens DL-3 utilizing rice hull. Bioresour. Technol. 99, 378–386. (doi:10.1016/j.biortech.2006.12.013) [DOI] [PubMed] [Google Scholar]
- 18.Jamroz D, Wiliczidewicz A, Skorupinska J. 1992. The effect of diets containing different levels of structural substances on morphological changes in the intestinal walls and the digestibility of the crude fiber fractions in geese. J. Anim. Feed. Sci. 1, 37–50. (doi:10.22358/jafs/69892/1992) [Google Scholar]
- 19.Yu B, Tsai CC, Hsu JC, Chiou PW. 1998. Effect of different sources of dietary fiber on growth performance, intestinal morphology and caecal carbohydrases of domestic geese. Br. Poult. Sci. 39, 560–567. (doi:10.1080/00071669888773) [DOI] [PubMed] [Google Scholar]
- 20.Yang J, Yang L, Wang Y, Zhai S, Wang S, Yang Z, Wang W. 2017. Effects of dietary protein and energy levels on digestive enzyme activities and electrolyte composition in the small intestinal fluid of geese. Anim. Sci. J. 88, 294–299. (doi:10.1111/asj.12557) [DOI] [PubMed] [Google Scholar]
- 21.Zhang SJ, Zhu CH, Guo J, Tang QP, Li HF, Zou JM. 2013. Metabolizable energy and fiber digestibility of uncommon feedstuffs for geese. Poult. Sci. 92, 1812–1817. (doi:10.3382/ps.2012-02515) [DOI] [PubMed] [Google Scholar]
- 22.Li M, Zhou H, Pan X, Xu T, Zhang Z, Zi X, Jiang Y. 2017. Cassava foliage affects the microbial diversity of Chinese indigenous geese caecum using 16S rRNA sequencing. Sci. Rep. 7, 45697 (doi:10.1038/srep45697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiao J, et al. 2014. In vitro evaluation on neutral detergent fiber and cellulose digestion by post-ruminal microorganisms in goats. J. Sci. Food. Agric. 94, 1745–1752. (doi:10.1002/jsfa.6485) [DOI] [PubMed] [Google Scholar]
- 24.Kashima Y, Udaka S. 2004. High-level production of hyperthermophilic cellulase in the Bacillus brevis expression and secretion system. Biosci. Biotechnol. Biochem. 68, 235–237. (doi:10.1271/bbb.68.235) [DOI] [PubMed] [Google Scholar]
- 25.Varga GA, Kolver ES. 1997. Microbial and animal limitations to fiber digestion and utilization. J. Nutrition. 127, 819S–823S. [DOI] [PubMed] [Google Scholar]
- 26.Castanon JIR. 2007. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 86, 2466–2471. (doi:10.3382/ps.2007-00249) [DOI] [PubMed] [Google Scholar]
- 27.Alloui MN, Szczurek W, Swiatkiewicz S. 2013. The usefulness of prebiotics and probiotics in modern poultry nutrition: a review. Ann. Anim. Sci. 13, 17–32. [Google Scholar]
- 28.Hong HA, Duc LH, Cutting SM. 2005. The use of bacterial spore formers as probiotics. FEMS. Microbiol. Rev. 29, 813–835. (doi:10.1016/j.femsre.2004.12.001) [DOI] [PubMed] [Google Scholar]
- 29.Ferreira CL, Salminen SJ, Grzeskowiak L, Brizuela MA, Sanchez L, Carneiro H, Bonnet M. 2011. Terminology concepts of probiotic and prebiotic and their role in human and animal health. Rev. Salud. Anim. 33, 137–146. [Google Scholar]
- 30.An BK, Cho BL, You SJ, Paik HD, Chang HI, Kim SW, Yun CW, Kang CW. 2008. Growth performance and antibody response of broiler chicks fed yeast derived β-glucan and single-strain probiotics. Asian-Australas. J. Anim. Sci. 21, 1027–1032. (doi:10.5713/ajas.2008.70571) [Google Scholar]
- 31.Aliakbarpour HR, Chamani M, Rahimi G, Sadeghi AA, Qujieq D. 2012. The Bacillus subtilis and lactic acid bacteria probiotics influences intestinal mucin gene expression, histomorphology and growth performance in broilers. Asian-Australas. J. Anim. Sci. 25, 1285–1293. (doi:10.5713/ajas.2012.12110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jerzsele A, Szeker K, Csizinszky R, Gere E, Jakab C, Mallo JJ, Galfi P. 2012. Efficacy of protected sodium butyrate, a protected blend of essential oils, their combination, and Bacillus amyloliquefaciens spore suspension against artificially induced necrotic enteritis in broilers. Poult. Sci. 91, 837–843. (doi:10.3382/ps.2011-01853) [DOI] [PubMed] [Google Scholar]
- 33.Huyghebaert G, Ducatelle R, Immerseel FV. 2011. An update alternatives to antimicrobial growth promoters for broilers. Vet. J. 187, 182–188. (doi:10.1016/j.tvjl.2010.03.003) [DOI] [PubMed] [Google Scholar]
- 34.Sun LH, Cao JY, Wang JJ, Guo PP, Wang ZG. 2017. Gene cloning and expression of cellulase of Bacillus amyloliquefaciens isolated from the cecum of goose. Anim. Biotechnol. 28, 74–82. (doi:10.1080/10495398.2016.1205594) [DOI] [PubMed] [Google Scholar]
- 35.Lynd LR, Wyman CE, Gemgross TU. 2002. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 66, 506–577. (doi:10.1128/MMBR.66.3.506-577.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lambertz C, Garvey M, Klinger J, Heesel D, Klose H, Fischer R, Commandeur U. 2014. Challenges and advances in the heterologous expression of cellulolytic enzymes: a review. Biotechnol. Biofuels. 7, 135 (doi:10.1186/s13068-014-0135-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xuan ZN, Kim JD, Heo KN, Jung HJ, Lee JH, Han YK, Kim YY, Han IK. 2001. Study on the development of a probiotics complex for weaned pigs. Asian-Australas J. Anim. Sci. 14, 1425–1428. (doi:10.5713/ajas.2001.1425) [Google Scholar]
- 38.Wei ZJ, Liao AM, Zhang HX, Liu J, Jiang ST. 2009. Optimization of supercritical carbon dioxide extraction of silkworm pupal oil applying the response surface methodology. Bioresour. Technol. 100, 4214–4219. (doi:10.1016/j.biortech.2009.04.010) [DOI] [PubMed] [Google Scholar]
- 39.Gessesse A, Mamo G. 1999. High-level xylanase production by an alkalophilic Bacillus sp. by using solid-state fermentation. Enzyme. Microb. Technol. 25, 68–72. (doi:10.1016/S0141-0229(99)00006-X) [Google Scholar]
- 40.Battan B, Sharma J, Dhiman SS, Kuhad RC. 2007. Enhanced production of cellulase-free thermostable xylanase by Bacillus pumilus ASH and its potential application in paper industry. Enzyme. Microb. Technol. 41, 733–739. (doi:10.1016/j.enzmictec.2007.06.006) [Google Scholar]
- 41.Sanghi A, Garg N, Kuhar K, Kuhar K, Kuhad RC, Gupta VK. 2009. Enhanced production of cellulase-free xylanase by alkalophilic Bacillus subtilis ASH and its application in biobleaching of kraft pulp. Bio. Resources. 4, 1109–1129. [Google Scholar]
- 42.Subramaniyan S, Sandhia GS, Prema P. 2001. Control of xylanase production without protease activity in Bacillus sp. by selection of nitrogen source. Biotechnol. Lett. 23, 369–371. (doi:10.1023/A:1005663704321) [Google Scholar]
- 43.Breccia JD, Sineniz F, Baigori MD, Hatti-Kaul R. 1998. Purification and characterization of a thermostable xylanase from Bacillus amyloliquefaciens. Enzyme. Microb. Technol. 22, 42–49. (doi:10.1016/S0141-0229(97)00102-6) [Google Scholar]
- 44.Kohli U, Nigam P, Singh D, Chaudhary K. 2001. Thermostable, alkalophilic and cellulase free xylanase production by Thermoactinomyces thalophilus subgroup C. Enzyme. Microb. Technol. 28, 606–610. (doi:10.1016/S0141-0229(01)00320-9) [DOI] [PubMed] [Google Scholar]
- 45.Dhillon A, Khanna S. 2000. Production of a thermostable alkali-tolerant xylanase from Bacillus circulans AB 16 grown on wheat straw. World. J. Microbiol. Biotechnol. 16, 325–327. (doi:10.1023/A:1008911026606) [Google Scholar]
- 46.Xi J, He LY, Huang Z, Sheng XF. 2014. Bacillus qingshengii sp. nov., a rock-weathering bacterium isolated from weathered rock surface. Int. J. Syst. Evol. Microbiol. 64, 2473–2479. (doi:10.1099/ijs.0.061929-0) [DOI] [PubMed] [Google Scholar]
- 47.Trivedi N, Gupta V, Kumar M, Kumari P, Reddy CR, Jha B. 2011. Solvent tolerant marine bacterium Bacillus aquimaris secreting organic solvent stable alkaline cellulase. Chemosphere 83, 706–712. (doi:10.1016/j.chemosphere.2011.02.006) [DOI] [PubMed] [Google Scholar]
- 48.Deka D, Jawed M, Goyal A. 2013. Purification and characterization of an alkaline cellulase produced by Bacillus subtilis (AS3). Prep. Biochem. Biotechnol. 43, 256–270. (doi:10.1080/10826068.2012.719849) [DOI] [PubMed] [Google Scholar]
- 49.Desriac N, Postollec F, Coroller L, Sohier D, Abee T, den Besten HM. 2013. Prediction of Bacillus weihenstephanensis acid resistance: the use of gene expression patterns to select potential biomarkers. Int. J. Food. Microbiol. 167, 80–86. (doi:10.1016/j.ijfoodmicro.2013.03.014) [DOI] [PubMed] [Google Scholar]
- 50.Le Lay J, Bahloul H, Sérino S, Jobin M, Schmitt P. 2015. Reducing activity, glucose metabolism and acid tolerance response of Bacillus cereus grown at various pH and oxydo-reduction potential levels. Food. Microbiol. 46, 314–321. (doi:10.1016/j.fm.2014.07.007) [DOI] [PubMed] [Google Scholar]
- 51.Gaggia F, Mattarelli P, Biavati B. 2010. Probiotics and prebiotics in animal feeding for safe food production. Int. J. Food. Microbiol. 141, S15–S28. (doi:10.1016/j.ijfoodmicro.2010.02.031) [DOI] [PubMed] [Google Scholar]
- 52.Novak KN, Davis E, Wehnes CA, Shields DR, Coalson JA, Smith AH, Rehberger TG. 2012. Effect of supplementation with an electrolyte containing a Bacillus-based direct-fed microbial on immune development in dairy calves. Res. Vet. Sci. 92, 427–434. (doi:10.1016/j.rvsc.2011.04.008) [DOI] [PubMed] [Google Scholar]
- 53.Sun P, Wang JQ, Zhang HT. 2010. Effects of Bacillus subtilis natto on performance and immune function of preweaning calves. J. Dairy. Sci. 93, 5851–5855. (doi:10.3168/jds.2010-3263) [DOI] [PubMed] [Google Scholar]
- 54.Zhu GY, Jiang YL. 2014. Polymorphism, genetic effect and association with egg production traits of chicken matrix metalloproteinases 9 promoter. Asian-Australas. J. Anim. Sci. 27, 1526–1531. (doi:10.5713/ajas.2014.14209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen W, Zhu XZ, Wang JP, Wang ZX, Huang YQ. 2014. Effects of Bacillus subtilis var. natto and Saccharomyces cerevisiae fermented liquid feed on growth performance, relative organ weight, intestinal microflora, and organ antioxidant status in Landes geese. J. Anim. Sci. 91, 978–985. (doi:10.2527/jas.2012-5148) [DOI] [PubMed] [Google Scholar]
- 56.Wu LY, Tan RB, Shi KJ. 2008. Effect of a dried Bacillus subtilis culture on gosling growth performance. Br. Poult. Sci. 49, 418–422. (doi:10.1080/00071660802213459) [DOI] [PubMed] [Google Scholar]
- 57.Lei K, Li YL, Yu DY, Rajput IR, Li WF. 2013. Influence of dietary inclusion of Bacillus licheniformis on laying performance, egg quality, antioxidant enzyme activities, and intestinal barrier function of laying hens. Poult. Sci. 92, 2389–2395. (doi:10.3382/ps.2012-02686) [DOI] [PubMed] [Google Scholar]
- 58.Abdelqader A, Irshaid R, Al-Fataftah AR. 2013. Effects of dietary probiotic inclusion on performance, eggshell quality, cecal microflora composition, and tibia traits of laying hens in the late phase of production. Trop. Anim. Health. Prod. 45, 1017–1024. (doi:10.1007/s11250-012-0326-7) [DOI] [PubMed] [Google Scholar]
- 59.Scheuermann SE. 1993. Effect of probiotic paciflor on energy and protein metabolism in growing pigs. Anim. Feed. Sci. Techonol. 41, 181–189. (doi:10.1016/0377-8401(93)90011-8) [Google Scholar]
- 60.Park SH, Perrotta A, Hanning I, Diaz-Sanchez S, Pendleton S, Alm E, Ricke SC. 2017. Pasture flock chicken cecal microbiome responses to prebiotics and plum fiber feed amendments. Poult. Sci. 96, 1820–1830. (doi:10.3382/ps/pew441) [DOI] [PubMed] [Google Scholar]
- 61.Lei XJ, Piao XS, Ru YJ, Zhang HY, Peron A, Zhang H. 2015. Effect of Bacillus amyloliquefaciens-based direct-fed microbial on performance, nutrient utilization, intestinal morphology and cecal microflora in broiler chickens. Asian-Australas. J. Anim. Sci. 28, 239–246. (doi:10.5713/ajas.14.0330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ye M, Sun L, Yang R, Wang Z, Qi K. 2017. Data from: The optimization of fermentation conditions for producing cellulase of Bacillus amyloliquefaciens and its application to goose feed Dryad Digital Repository. (doi:10.5061/dryad.27j11) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Ye M, Sun L, Yang R, Wang Z, Qi K. 2017. Data from: The optimization of fermentation conditions for producing cellulase of Bacillus amyloliquefaciens and its application to goose feed Dryad Digital Repository. (doi:10.5061/dryad.27j11) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data supporting this article are available on Dryad: (https://doi.org/10.5061/dryad.27j11) [62].