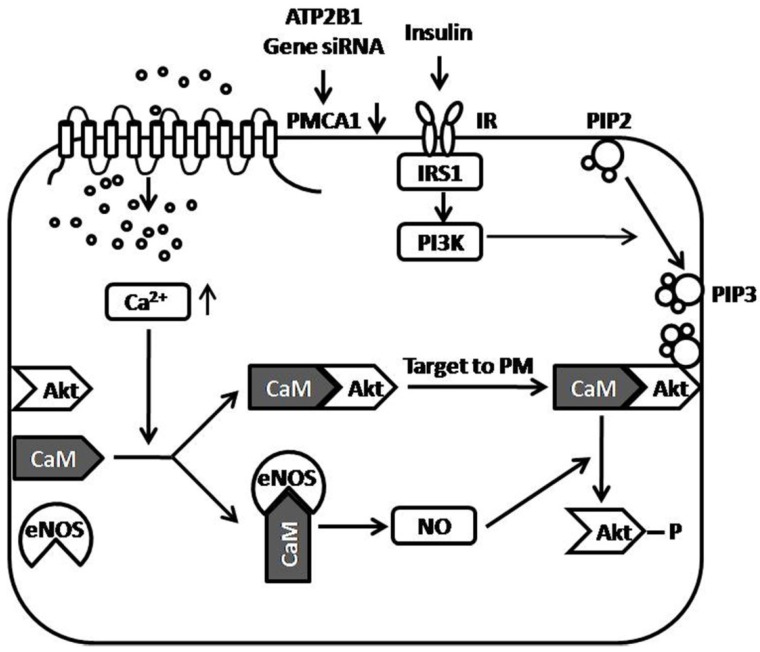
Figure 5.
Proposed model of the role of the PMCA1 protein in regulating insulin-induced Akt phosphorylation. ATP2B1 gene silencing, which results in decreased expression of the PMCA1 protein, leads to enhanced intracellular Ca2+ concentrations in endothelial cells. Enhanced Ca2+ concentrations by ATP2B1 gene silencing facilitate insulin-induced Akt activation through Ca2+/calmodulin signaling pathway. Moreover, ATP2B1 gene silencing results in greater insulin-induced eNOS activity via the Ca2+/calmodulin signaling pathway. Additionally, the Ca2+-associated activation of eNOS was involved in modifying insulin sensitivity in ATP2B1-silenced HUVECs. ATP2B1: ATPase plasma membrane Ca2+ transporting 1; PMCA1: Plasma Membrane Calcium ATPase 1; IR: insulin receptor; IRS1: insulin receptor substrate 1; CaM: calmodulin; Akt: protein kinase B; eNOS: endothelial nitric oxide synthase; NO: nitric oxide; PM: plasma membrane; PIP2: Phosphatidylinositol-4, 5-biphosphate; PIP3: Phosphatidylinositol-3, 4, 5-triphosphate; PI3K: Phosphatidylinositol 3-Kinase.