Abstract
Bordetella pertussis and Bordetella parapertussis are the causal agents of whooping cough in humans. They produce diverse virulence factors, including adenylate cyclase-hemolysin (AC-Hly), a secreted toxin of the repeat in toxins (RTX) family with cyclase, pore-forming, and hemolytic activities. Post-translational modifications (PTMs) are essential for the biological activities of the toxin produced by B. pertussis. In this study, we compared AC-Hly toxins from various clinical isolates of B. pertussis and B. parapertussis, focusing on (i) the genomic sequences of cyaA genes, (ii) the PTMs of partially purified AC-Hly, and (iii) the cytotoxic activity of the various AC-Hly toxins. The genes encoding the AC-Hly toxins of B. pertussis and B. parapertussis displayed very limited polymorphism in each species. Most of the sequence differences between the two species were found in the C-terminal part of the protein. Both toxins harbored PTMs, mostly corresponding to palmitoylations of the lysine 860 residue and palmoylations and myristoylations of lysine 983 for B. pertussis and AC-Hly and palmitoylations of lysine 894 and myristoylations of lysine 1017 for B. parapertussis AC-Hly. Purified AC-Hly from B. pertussis was cytotoxic to macrophages, whereas that from B. parapertussis was not.
Keywords: adenylate cyclase hemolysin, Bordetella pertussis, Bordetella parapertussis, PTMs
1. Introduction
Whooping cough is a vaccine-preventable disease caused by Bordetella pertussis or Bordetella parapertussis in humans. Both these pathogens produce many virulence factors [1]: adhesins, such as filamentous hemagglutinin (FHA) and pertactin (PRN), and toxins, such as pertussis toxin (PT), which is produced specifically by B. pertussis, and adenylate cyclase hemolysin (AC-Hly). As for other Bordetella virulence factors [2], the expression of these factors is regulated by Bvg [2]. Moreover, as recently shown in B. pertussis, the expression of PT and AC-Hly is also modulated by RpoE sigma factor [3]. B. pertussis AC-Hly (Bp-AC-Hly) is a 1706-amino acid protein (Uniprot P0DKX7) with an adenylate cyclase (AC) domain in its first 400 amino acids and a 1306-amino acid repeat in toxins (RTX) domain consisting of hydrophobic pore-forming (500–700), fatty acyl-modified (800–1000), calcium-binding (1000–1600), and C-terminal secretion signal subdomains. The AC-Hly of B. parapertussis (Bpp-AC-Hly) has a similar structure [4,5]. These proteins are encoded by the cyaA gene (BP0760 in B. pertussis and BPP0321 in B. parapertussis).
The Bp-AC-Hly protein has a complex structure and multiple activities [6,7,8,9]: (i) binding to the host cell, (ii) translocation of the enzymatic domain (AC), resulting in supraphysiological intracellular cyclic adenosine monophosphate (cAMP) levels after calmodulin activation, and (iii) Ca2+-dependent pore-forming activity on target cells [10,11]. Skopova et al. [12] recently used different B. pertussis AC-Hly mutants to demonstrate that the ability of Bp-AC-Hly to increase cAMP levels was sufficient for lung infection, but both AC and pore-forming activities were required for full virulence. Bp-AC-Hly and Bpp-AC-Hly must undergo post-translational modifications (PTMs) for activity. These PTMs are mediated by an acyl transferase encoded by the cyaC gene, which is co-expressed with cyaA [5]. Two PTM sites have been identified in Bp-AC-Hly: the lysine residues in positions 860 and 983 [13,14,15,16] according to the PTM database (http://dbptm.mbc.nctu.edu.tw). The lysine 983 residue of Bp-AC-Hly is palmitoylated, but the nature of the modification to this residue in Bpp-AC-Hly is unknown. Both Bp- and Bpp-AC-Hly induce protective immunity in the murine respiratory model [17,18,19,20].
AC-Hly is difficult to purify from B. pertussis cultures. Moreover, Bp-AC-Hly is a hydrophobic protein that aggregates or is degraded during purification. Its solubilization requires a denaturing agent, such as urea. For these reasons, most structural, biological, and immunological studies of Bp-AC-Hly have used the recombinant Bp-AC-Hly. This molecule is produced in E. coli K-12 harboring the B. pertussis 18323-cyaA and 18323-cyaC genes, encoding the toxin and the enzyme required to the appropriate modifications on the toxin, respectively [21]. However, the B. pertussis 18323 strain belongs to a different genomic clade from the other B. pertussis isolates circulating around the world [22,23]. In addition, the recombinant Bp-AC-Hly produced in E. coli harbors different PTMs and has different biological and protective activities from those of the toxin produced directly by B. pertussis isolates [14,24].
Over the last four decades, B. pertussis and B. parapertussis genomes have been shown to evolve under vaccination- and disease-induced pressure, adapting to human populations [22,23,25,26,27,28]. The genomes of B. pertussis and B. parapertussis contain different insertion sequences, mostly IS481 for B. pertussis and IS1001 for B. parapertussis, and these sequences make a major contribution to the deletion, insertion, or inactivation of genes, as observed in some B. pertussis isolates displaying IS481 insertions within the prn gene [29]. The gene encoding Bp-AC-Hly has never been deleted or inactivated in any B. pertussis or B. parapertussis isolates, whereas the corresponding Bb-AC-Hly cyaA gene of some B. bronchiseptica isolates can be replaced with a peptide transport protein operon [23,30]. Only one clinical isolate of B. pertussis presenting a duplication of cyaA has been described to date [31].
Given the important role of AC-Hly in the pathogenesis of B. pertussis, many experts around the world have proposed the inclusion of recombinant AC-Hly in pertussis acellular vaccines [8]. In this context, we felt that it was important (i) to compare the B. pertussis and B. parapertussis cyaA genes; (ii) to compare the PTMs of the AC-Hly toxins produced by various B. pertussis isolates with different properties; (iii) to characterize and compare the PTMs of Bpp-AC-Hly toxins produced by various B. parapertussis isolates; and (iv) to compare the cytotoxic activities of Bp- and Bpp-AC-Hly.
2. Results
2.1. Genomic Analysis of the B. pertussis and B. parapertussis cyaA Genes
We analyzed polymorphism of the cyaA gene using the Bordetella virulence-associated genes scheme of the Bordetella MLST database and considering the locus BORD005031 corresponding to cyaA (https://pubmlst.org/bordetella/). This locus has 47 alleles in the various Bordetella species producing AC-Hly for which data are recorded in the database: 35 alleles from B. bronchiseptica, 3 from B. parapertussis (alleles 3, 5, and 37), and 9 from B. pertussis (alleles 1, 4, 7, 8, 9, 42, 43, 44, and 47). Figure S1 presents the polymorphisms of the 47 different cyaA alleles.
Focusing on the nine alleles from B. pertussis and considering the 603 isolates for which data are publicly available for this locus in the database, we found that 90.0% of the isolates (i.e., 543/603) had allele 4, as in the Tohama reference strain; 7.6% (i.e., 41/603) had allele 7, as in the 18323 reference strain; 1.5% (i.e., 9/603) had allele 9; 0.9% (i.e., 6/603) had allele 42; and the remaining alleles (8, 43, 44, and 47) were each present in a single isolate (1/603). Four of these nine alleles are synonymous and five encode proteins with amino-acid substitutions. Non-synonymous alleles are nevertheless infrequent, because such alleles were found in only 2.9% (18/603) of isolates, most of which were collected between 1960 and 1995. Data for only five isolates of B. parapertussis were publicly available from the Bordetella MLST database, including the human reference strain Bpp12822 and the ovine reference strain Bpp5. Only three different alleles were identified for B. parapertussis: two in human isolates (alleles 3 and 5) and one in an ovine isolate (allele 37). We extended this analysis to recently obtained human B. parapertussis isolates, which were also all found to contain allele 3, illustrating the high degree of conservation of the cyaA gene in B. parapertussis isolates (data not shown). We found that two polymorphic sites resulting in amino-acid substitutions were specific to allele 37 of the ovine Bpp5 (M37V and S560A) strain, one was specific to allele 5 (T1139S), and two others were specific to human isolates (i.e., V567G and G1249D) (Figure S1). A more detailed examination of Figure S1 reveals that some of the nonsynonymous polymorphisms found in B. parapertussis are also common to B. bronchiseptica; there are four such polymorphisms in the sequence encoding amino acids 800 to 1000 of the protein and 29 in the sequence encoding amino acids 1000–1600 of the protein. This is in agreement with previous reports [4,9,32]. These findings indicate that the Bpp-cyaA gene is closer to Bb-cyaA than to Bp-cyaA, and that there are large differences in this part of the protein between Bp-AC-Hly and the other two proteins.
2.2. Partial Purification of AC-Hly
Extraction with urea and by affinity chromatography on a calmodulin column resulted in similar purification yields for all isolates: 1.4 ± 0.4 mg/mL for all B. pertussis isolates. Similar amounts of protein (1.5 ± 0.3 mg/mL) were obtained from PRN-deficient B. pertussis isolates, but yields were lower for FHA-deficient B. pertussis isolates (0.9 ± 0.3 mg/mL). Similar amounts of protein were obtained from all B. parapertussis isolates (1.3 ± 0.4 mg/mL) and PRN-deficient B. parapertussis isolates (1.1 ± 0.1 mg/mL).
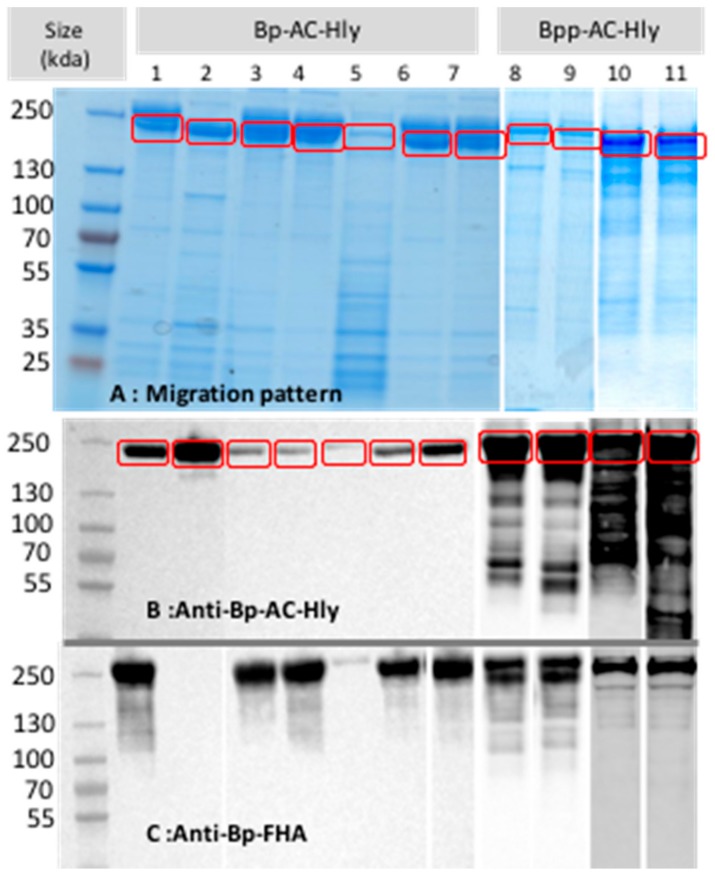
The purified AC-Hly preparations contained several additional proteins (Figure 1A); some were proteolysis fragments, as shown by Western blotting with a polyclonal anti-AC-Hly serum (Figure 1B), whereas others were contaminants, including FHA (Figure 1C). The Bpp-AC-Hly were more proteolyzed than the Bp-AC-Hly.
Figure 1.
(A) Protein migration pattern, (B) Western blot with a polyclonal anti-Bp-AC-Hly antibody, (C) Western blot with a polyclonal anti-Bp-FHA antibody (lane 1: Tohama, lane 2: FR4624, lane 3: FR5133, lane 4: FR5187, lane 5: CIP1672, lane 6: FR5388, lane 7: FR5392, lane 8: Bpp12822, lane 9: BPP1, lane 10: FR3728, lane 11: FR5840) Full length AC-Hly is surrounded. Bp = B. pertussis; Bpp = B. parapertussis; AC-Hly = adenylate cyclase hemolysin; and FHA = filamentous hemagglutinin.
2.3. Post-Translational Modifications
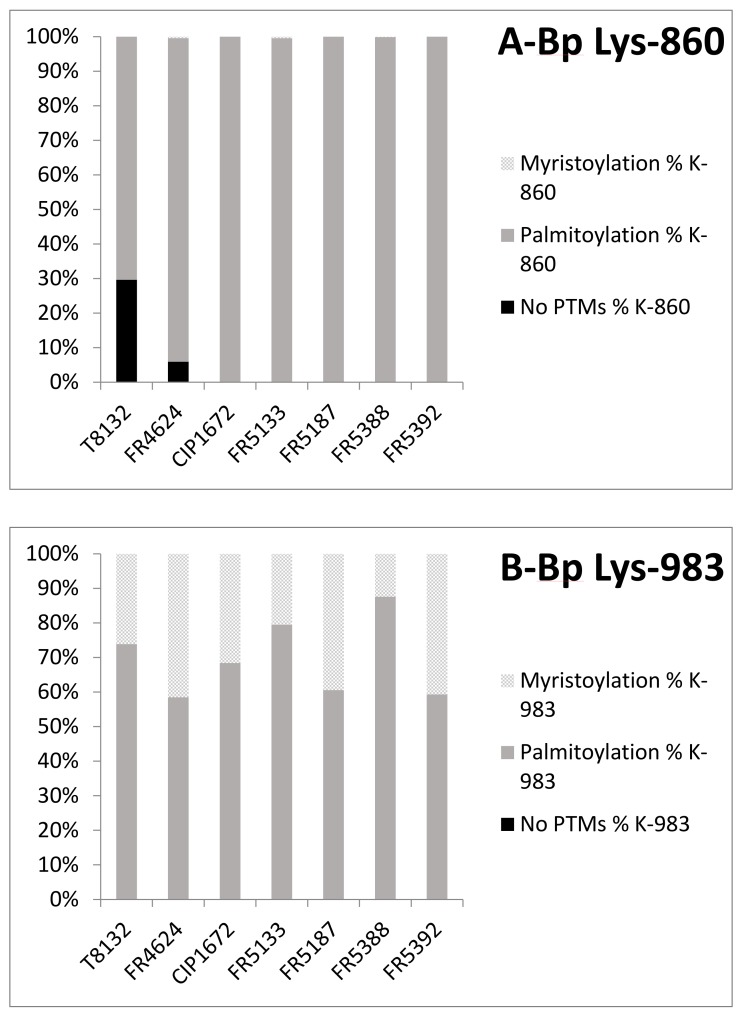
A mass spectrometry analysis was done on purified Bp-AC-Hly and Bpp-AC-Hly after tryptic digestion (Fragmentation spectra are presented in Figures S2 and S3). This analysis confirmed that Bp-AC-Hly underwent post-translational modifications. The following PTMs were observed for all the purified preparations of Bp-AC-Hly: a palmitoyl group on lysine 860 and a palmitoyl or a myristate group on lysine 983 (Figure 2).
Figure 2.
Post-translational modifications (PTMs) observed for Bp-Ac-Hly ((A) PTMs on K-860; (B) PTMs on K-983). From the modifications Specific Peptides output table of the MaxQuant results, intensities of the unmodified and modified peptides of interest (peptide forms) were extracted. For each lysine residue (K-860 and K-983), intensities were summed by peptide form and normalized against the sum of all extracted intensities per involved lysine. These calculated percentages were plotted on the bar chart representation: Myristoylation (light grey), Palmitoyalation (intermediate grey), no PTMs (dark). The intensities of peptide ions used in the bar charts (Figure 2 and Figure 3) do not necessarily directly correlate with the actual amount of each corresponding peptide since ionization efficiencies can vary with the presence of post-translational modifications. However, myristoylation and palmitoylation are comparable modifications that occur on the same amino acid (Lysine), and, thus, we can postulate that the ionization efficiencies of the corresponding peptides should not be very different.
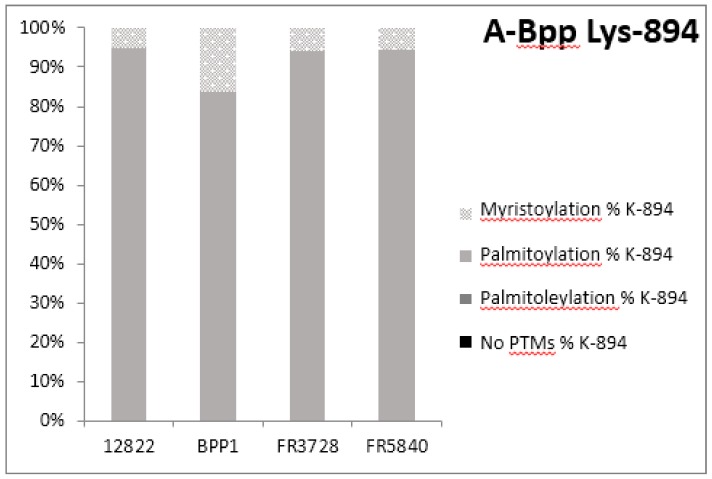
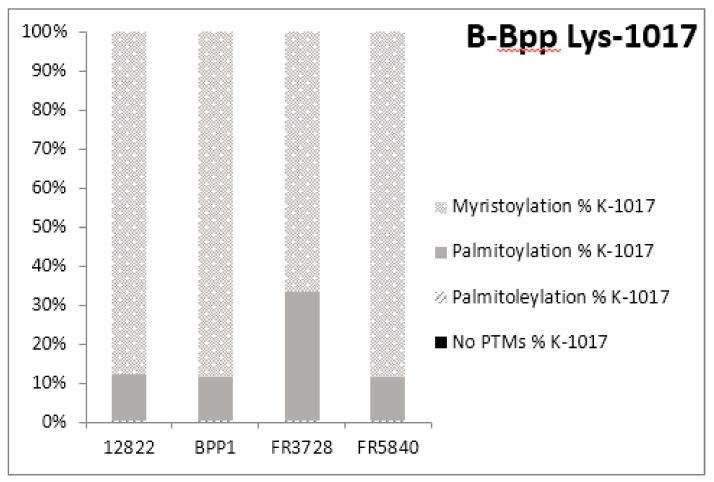
PTMs were also detected in the various purified preparations of Bpp-AC-Hly: mostly palmitoylation of lysine 894 and myristoylation on lysine 1017 (Figure 3).
Figure 3.
PTMs observed for Bpp-Ac-Hly ((A) PTMs on K-894; (B) PTMs on K-1017). From the modifications Specific Peptides output table of the MaxQuant results, intensities of the unmodified and modified peptides of interest (peptide forms) were extracted. For each lysine residue (K-894 and K-1017), intensities were summed by peptide form and normalized against the sum of all extracted intensities per involved lysine. These calculated percentages were plotted on the bar chart representation: Myristoylation (light grey), Palmitoyalation (intermediate grey), Palmitoleylation (hatched), no PTMs (dark). The intensities of peptide ions used in the bar charts (Figure 2 and Figure 3) do not necessarily directly correlate with the actual amount of each corresponding peptide since ionization efficiencies can vary with the presence of post-translational modifications. However, myristoylation, palmitoylation, and palmitoleylation are comparable modifications that occur on the same amino acid (Lysine), and, thus, we can postulate that the ionization efficiencies of the corresponding peptides should not be very different.
2.4. Cytotoxicity toward Macrophages
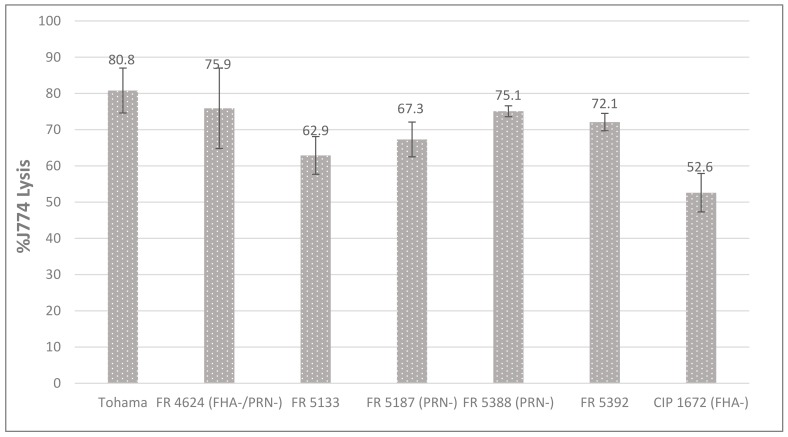
We evaluated the cytotoxicity of all purified preparations of AC-Hly with J774.A1 macrophages. As shown in Figure 4 for B. pertussis, all Bp-AC-Hly toxins were similarly cytotoxic to these cells, whereas no cytotoxicity was observed for any of the Bpp-AC-Hly toxins (Figure S4).
Figure 4.
Percentage of J774.A1 cells lysed by Bp-AC-Hly. The results presented are the mean ± standard deviation of three different experiments.
3. Discussion
The AC-Hly protein is encoded by the cyaA gene (BP0760 in B. pertussis, BPP0321 in B. parapertussis). Unlike other virulence genes encoding vaccine antigen proteins displaying allelic variation (such as the ptxA and prn genes), cyaA is stable, with few polymorphisms detected in the two species. Most B. pertussis isolates carried the same cyaA allele as the Tohama reference strain (i.e., allele 4 in the PubMLST database) and all human B. parapertussis isolates carried the same allele as the 12,822 reference strain (i.e., allele 3). This finding is consistent with the results of the SNP study by Bart et al. [22], which reported only two different SNPs within cyaA, relative to the Tohama strain, in only seven isolates (corresponding to alleles 7 and 4) from a set of more than 300 isolates collected from around the world in different vaccination eras. Our findings are also consistent with those of Chenal-Francisque et al. [32], who found no polymorphism in the C-terminal part of cyaA in various B. pertussis and B. parapertussis isolates. However, there are a large number of sequence differences between the cyaA genes of B. pertussis and those of B. parapertussis, mostly affecting amino-acids 800–1000 and 1000–1600 of the protein, corresponding to the region carrying the protective epitope and receptor-binding domain sites for human cells. Based on MLST, B. pertussis and B. parapertussis belong to two different complexes that evolved recently and independently, from two different B. bronchiseptica complexes [28].
Despite the low purification yields for native AC-Hly, this step is a prerequisite for the study of native Bp-AC-Hly and Bpp-AC-Hly. We purified AC-Hly from cultures of various B. pertussis and B. parapertussis isolates, some of which did not produce PRN or FHA. Production and purification yields were similar for all isolates, regardless of whether they produced PRN. Smaller amounts of Bp-AC-Hly were obtained from FHA-deficient isolates which correlates with the observations of Zaretzky et al. [33]. The presence of residual FHA was detected in all Bp-AC-Hly and Bpp-AC-Hly preparations except those originating from FHA-deficient B. pertussis isolates. This finding is consistent with the already demonstrated strong interaction between FHA and AC-Hly: (i) FHA is thought to play a role in the surface retention of AC-Hly toxin through a physical interaction, and AC-Hly enhances the adhesin functions of FHA [33,34]; (ii) an interaction between FHA and Bp-AC-Hly was recently shown to inhibit biofilm formation in vitro [35]; (iii) the absence of FHA has been reported to lead to the enhanced expression of other virulence factors in B. pertussis isolates [36]; and (iv) the inhibition of AC-induced macrophage lysis by anti-FHA antibodies seems to be modified in recent isolates [37].
The activity of AC-Hly is dependent on PTMs. In 1994, Hackett et al. [13] demonstrated that Bp-AC-Hly (produced from the Bp338 strain, a derivative of the Tohama reference strain) displayed 100% acylation of the Lys 983 residue involving the addition of palmitoyl. The following year [14], they showed that the PTMs observed on a recombinant Bp-AC-Hly with different biological and protective activities, were not the same; the Lys 983 residue of the recombinant toxin was acylated with mainly palmitate and some miristate. Furthermore, most of the lysine 860 residues were also palmitoylated. We also detected a mixture of palmitoyl and myristoyl acylations on the lysine 983 residue of the Tohama AC-Hly, as observed for the recombinant Bp-AC-Hly, but not in the first study by Hackett et al. [13]. Myristate was also observed on the Bp-AC-Hly produced by the other isolates Furthermore, by contrast to the initial study published by Hackett [13], we observed an acylation of the lysine 860 residue in Bp-AC-Hly purified from the Tohama reference strain, as in AC-Hly from other isolates. This acylation was mostly palmitoyl, as already reported for the recombinant Bp-AC-Hly. Our preliminary results indicate that the modifications of the toxins produced by various B. pertussis isolates are similar to those of the recombinant toxin. These observations suggest that the differences in biological and protective activities between the recombinant toxin and the toxins produced by B. pertussis are not due to differences in PTMs. We suggest instead that these differences are mostly due to interactions with other components of B. pertussis. FHA may be one of the components involved in these interactions, possibly together with PRN and lipopolysaccharide (LPS), which have also been identified as potentially important [33,37,38,39,40,41]. The interactions between FHA and AC-Hly and those with PRN may be crucial for the final conformation of AC-Hly and for its immunogenic and protective activities. Additional studies are required to evaluate the protective activity of recombinant Bp-AC-Hly, because its conformation may be suboptimal relative to that of the Bp-AC-Hly produced and secreted by B. pertussis.
In this study, we found that the PTMs of Bpp-AC-Hly concerned the lysine residues in positions 894 and 1017. These modifications were essentially the palmitoylation of Lys 894, as for the Lys 860 residue of B. pertussis. The Lys 1017 residue was mostly myristoylated, but some palmitoylation was observed, contrary to the findings for Bp-AC-Hly. In addition, we observed that contrary to Bp-AC-Hly, none of Bpp-AC-Hly were cytotoxic for macrophages. This is in agreement with previous data obtained from direct bacteria-cell interaction cytotoxicity tests [26]. We hypothesize that the lack of cytotoxicity of Bpp-AC-Hly may reflect not only differences in PTMs, but also differences in interactions with FHA or LPS, particularly as LPS differs between Bordetella species [42,43,44]. In particular, the Bpp LPS contains an O-antigen, whereas the Bp LPS does not. Further studies are required to clarify this point.
In conclusion, our study confirms that AC-Hly is a toxin that is highly conserved in both B. pertussis and B. parapertussis, unlike other virulence factors, confirming its important role in the pathogenesis of whooping cough.
4. Materials and Methods
4.1. Cultures of Bacteria
We selected seven B. pertussis isolates and four B. parapertussis isolates for study, including the reference strains Tohama CIP8132 and Bpp12822. The characteristics of the isolates are presented in Table 1.
Table 1.
Characteristics of the isolates. PRN = pertactin.
| Name | Species | Year of Collection | Antigen Deficiency | References |
|---|---|---|---|---|
| Tohama(CIP8132) | B. pertussis | 1954 | none | [29] |
| FR4624 | B. pertussis | 2009 | FHA/PRN | [45] |
| 1672 | B. pertussis | 1950 | FHA | [37,45] |
| FR5133 | B. pertussis | 2012 | none | [37] |
| FR5187 | B. pertussis | 2012 | PRN | [37] |
| FR5388 | B. pertussis | 2012 | PRN | [37] |
| FR5392 | B. pertussis | 2012 | none | [37] |
| Bpp12822 | B. parapertussis | 1993 | none | [26] |
| BPP1 | B. parapertussis | 1990 | none | [26] |
| FR3728 | B. parapertussis | 2007 | PRN | [26] |
| FR5840 | B. parapertussis | 2014 | PRN | this study |
All isolates were grown on Bordet Gengou agar (BGA) (Difco, Franklin Lakes, NJ, USA,) supplemented with 15% defibrinated sheep blood, at 36 °C for 72 h. Isolates were re-plated and incubated for another 24 h before use in liquid culture and AC-Hly purification or cytotoxicity assays, as previously described [37].
4.2. AC-Hly Purification
After growth on a BGA plate and a first liquid preculture in Stainer and Scholte medium [46], isolates were further subcultured in 8 × 400 mL of medium, until their optical density increased from an OD650 0.2 to an OD650 1 ± 0.2. Cultures were then centrifugated at 9000× g for 25 min at 4 °C. The bacterial pellet was weighed, suspended in a Tris 50mM/CaCl2 0,22mM − Hepes20mM − Urea 5M/pH = 8 buffer (using 5 mL/g of pellet) on ice and incubated with continuous shaking at +4 °C for 1 h. The suspension was then centrifuged at 17,000× g for 30 min at 4 °C. AC-Hly was purified on calmodulin agarose, as previously described [47]. Eluates were concentrated with Amicon Ultra-15 and Ultra-2 devices (Merck Millipore, Billerica, MA, USA). Protein concentration was determined with a Qubit 3.0 Fluorometer (Life technologies, Carlsbad, CA, USA). Purification yields are expressed as mg/mL of concentrated eluate.
4.3. Western Blots
Western blot analyses were performed as previously described [48], with specific polyclonal antibodies directed against highly purified recombinant Bp-AC-Hly (a gift from P. Sebo) or highly purified Bp-FHA (a gift from GSK).
4.4. Cytotoxicity Assays
J774.A1 murine monocyte/macrophage-like cells (ATCC ref TIB-67) were cultured and cytotoxicity assays were performed as previously described [29]. Cytotoxicity/inhibition assays were performed as described elsewhere [37].
4.5. PTM Determination
Each purified protein solution was adjusted to a concentration of 4.4 M urea with 50 mM Tris-HCl pH 7.6 buffer, and 20 µg of protein was reduced in 5 mM TCEP (Sigma, St. Louis, MO, USA) and alkylated in 25 mM iodoacetamide (Sigma). Proteins were consecutively digested with rLys-C (Promega, Madison, WI, USA) and Sequencing-Grade Modified Trypsin (Promega, Madison, WI, USA) in a 1:50 ratio (enzyme:protein). The digested peptides were purified on Pierce C18 Spin Columns (Thermo Fisher Scientific, Waltham, MA, USA). Peptides were eluted in 90% ACN/0.1% FA, dried in a Speedvac, and resuspended in 2% ACN/0.1% FA.
The digests were analyzed with an Ultimate 3000 nano-HPLC (Dionex, Sunnyvale, CA, USA) coupled online to an LTQ-Orbitrap Velos Mass Spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) equipped with an ETD source. We loaded 0.5 μg of peptides onto a C4 trap column (Acclaim™ PepMap™ 300, 5 μm, 300 Å, 300 μm i.d. × 5 mm—Thermo Fisher Scientific, Waltham, MA, USA) and separated them on a custom-built 20 cm C4 column (5 μm particles, 300 Å pore size, ReproSil-Pur 300 C4, Dr. Maisch GmbH, Ammerbuch-Entringen, Germany) with a one-step gradient from 2.0% to 90% solvent B (80% ACN/0.1% FA) over 43 min, at a flow rate of 300 nL/min over 78 min. Mass spectrometry (MS) data were acquired with Xcalibur software in survey scan (300–1700 m/z) mode and were analyzed in the Orbitrap mass analyzer at a resolution of 60,000. Five ETD fragmentations were then analyzed in the linear ion trap. The AGC targets for MS and MS/MS scans were set to 1E6 and 5E3, respectively. The isolation width was set at 2.0 m/z and the activation time was set at 150 ms. Selected ions were dynamically excluded for 20 s.
Raw data were analyzed with MaxQuant software version 1.5.1.2 [49] and the Andromeda search engine [50] against the Bordetella pertussis and Bordetella parapertussis UniProt databases, containing 3258 proteins and 4161 proteins, respectively. The digestion mode was set to trypsin and a maximum of two missed cleavages were allowed. N-terminal acetylation and methionine oxidation were allowed as variable modifications and cysteine carbamidomethylation as a fixed modification. For the PTM analysis, additional N-acetylated lysines (myristoyl-4H, myristoleylation, myristoylation, palmitoleylation, palmitoylation) were considered as variable modifications. The minimum peptide length was fixed at five amino acids and the required false discovery rate was set at 1% for PSMs and proteins. The main search peptide tolerance was set at 4.5 ppm and 0.5 Da for the MS/MS match tolerance. Second peptides were used to identify co-fragmentation events and to match, between runs, an accepted match time window of 0.7 min for an alignment time window of 20 min.
From the modifications SpecificPeptides output table of the MaxQuant result, we extracted the intensities of the unmodified and modified peptides of interest (peptide forms). For a given lysine residue, intensities were summed by peptide form and normalized against the sum of all extracted intensities per involved lysine. Bar charts were created with these values (Figure 2A,B and Figure 3A,B).
Acknowledgments
We thank Nicolas Hegerle for his help at the start of this study. This work was supported by the Institut Pasteur Foundation and GlaxoSmithKline Biologicals, Rixensart, Belgium.
Supplementary Materials
The following are available online at www.mdpi.com/2072-6651/9/10/304/s1. Figure S1: Translate—aligned protein sequences BORD005031 (cyaA), obtained from Locus Explorer—Bordetella MLST locus/sequence definitions (https://pubmlst.org/bordetella/); Figure S2: Annotated MaxQuant MS/MS spectrum corresponding to the peptide containing the modified Lysines for Bp-AC-Hly; Figure S3: Annotated MaxQuant MS/MS spectrum corresponding to the peptide containing the modified Lysines for Bpp-AC-Hly; Figure S4: Percentage of J774.A1 cells lysed by Bpp-AC-Hly. The results presented are the mean ± standard deviation of different experiments.
Author Contributions
V.B. and N.G. conceived and designed the experiments; S.D. and M.D. performed the cultures, protein purification, and analysis; T.D., M.M., and J.C.-R. designed the PTMs analysis; T.D. performed the PTMs analysis; T.D. and M.M. analyzed and validated the PTM data; V.B. compiled all the data; V.B. and N.G. wrote the paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Guiso N., Hegerle N. Other bordetellas, lessons for and from pertussis vaccines. Expert Rev. Vaccines. 2014;13:1125–1133. doi: 10.1586/14760584.2014.942221. [DOI] [PubMed] [Google Scholar]
- 2.Scarlato V., Prugnola A., Arico B., Rappuoli R. Positive transcriptional feedback at the bvg locus controls expression of virulence factors in Bordetella pertussis. Proc. Nat. Acad. Sci. USA. 1990;87:6753–6757. doi: 10.1073/pnas.87.17.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbier M., Boehm D.T., Sen-Kilic E., Bonnin C., Pinheiro T., Hoffman C., Gray M., Hewlett E., Damron F.H. Modulation of pertussis and adenylate cyclase toxins by sigma factor rpoe in Bordetella pertussis. Infect. Immun. 2017;85 doi: 10.1128/IAI.00565-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betsou F., Sismeiro O., Danchin A., Guiso N. Cloning and sequence of the bordetella bronchiseptica adenylate cyclase-hemolysin-encoding gene: Comparison with the Bordetella pertussis gene. Gene. 1995;162:165–166. doi: 10.1016/0378-1119(95)00339-8. [DOI] [PubMed] [Google Scholar]
- 5.Parkhill J., Sebaihia M., Preston A., Murphy L.D., Thomson N., Harris D.E., Holden M.T., Churcher C.M., Bentley S.D., Mungall K.L., et al. Comparative analysis of the genome sequences of Bordetella pertussis, bordetella parapertussis and bordetella bronchiseptica. Nat. Genet. 2003;35:32–40. doi: 10.1038/ng1227. [DOI] [PubMed] [Google Scholar]
- 6.Masin J., Osicka R., Bumba L., Sebo P. Bordetella adenylate cyclase toxin: A unique combination of a pore-forming moiety with a cell-invading adenylate cyclase enzyme. Pathog. Dis. 2015;73:ftv075. doi: 10.1093/femspd/ftv075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carbonetti N.H. Pertussis toxin and adenylate cyclase toxin: Key virulence factors of Bordetella pertussis and cell biology tools. Future Microbiol. 2010;5:455–469. doi: 10.2217/fmb.09.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sebo P., Osicka R., Masin J. Adenylate cyclase toxin-hemolysin relevance for pertussis vaccines. Expert Rev. Vaccines. 2014;13:1215–1227. doi: 10.1586/14760584.2014.944900. [DOI] [PubMed] [Google Scholar]
- 9.Guiso N. Bordetella adenylate cyclase-hemolysin toxins. Toxins. 2017;9:277. doi: 10.3390/toxins9090277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiser R., Masin J., Bumba L., Pospisilova E., Fayolle C., Basler M., Sadilkova L., Adkins I., Kamanova J., Cerny J., et al. Calcium influx rescues adenylate cyclase-hemolysin from rapid cell membrane removal and enables phagocyte permeabilization by toxin pores. PLoS Pathog. 2012;8:e1002580. doi: 10.1371/journal.ppat.1002580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sotomayor-Perez A.C., Ladant D., Chenal A. Calcium-induced folding of intrinsically disordered repeat-in-toxin (rtx) motifs via changes of protein charges and oligomerization states. J. Biol. Chem. 2011;286:16997–17004. doi: 10.1074/jbc.M110.210393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skopova K., Tomalova B., Kanchev I., Rossmann P., Svedova M., Adkins I., Bibova I., Tomala J., Masin J., Guiso N., et al. Cyclic amp-elevating capacity of adenylate cyclase toxin-hemolysin is sufficient for lung infection but not for full virulence of Bordetella pertussis. Infect. Immun. 2017;85 doi: 10.1128/IAI.00937-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hackett M., Guo L., Shabanowitz J., Hunt D.F., Hewlett E.L. Internal lysine palmitoylation in adenylate cyclase toxin from Bordetella pertussis. Science. 1994;266:433–435. doi: 10.1126/science.7939682. [DOI] [PubMed] [Google Scholar]
- 14.Hackett M., Walker C.B., Guo L., Gray M.C., Van Cuyk S., Ullmann A., Shabanowitz J., Hunt D.F., Hewlett E.L., Sebo P. Hemolytic, but not cell-invasive activity, of adenylate cyclase toxin is selectively affected by differential fatty-acylation in escherichia coli. J. Biol. Chem. 1995;270:20250–20253. doi: 10.1074/jbc.270.35.20250. [DOI] [PubMed] [Google Scholar]
- 15.Basar T., Havlicek V., Bezouskova S., Hackett M., Sebo P. Acylation of lysine 983 is sufficient for toxin activity of Bordetella pertussis adenylate cyclase. Substitutions of alanine 140 modulate acylation site selectivity of the toxin acyltransferase cyaC. J. Biol. Chem. 2001;276:348–354. doi: 10.1074/jbc.M006463200. [DOI] [PubMed] [Google Scholar]
- 16.Basar T., Havlicek V., Bezouskova S., Halada P., Hackett M., Sebo P. The conserved lysine 860 in the additional fatty-acylation site of Bordetella pertussis adenylate cyclase is crucial for toxin function independently of its acylation status. J. Biol. Chem. 1999;274:10777–10783. doi: 10.1074/jbc.274.16.10777. [DOI] [PubMed] [Google Scholar]
- 17.Betsou F., Sebo P., Guiso N. The C-terminal domain is essential for protective activity of the Bordetella pertussis adenylate cyclase-hemolysin. Infect. Immun. 1995;63:3309–3315. doi: 10.1128/iai.63.9.3309-3315.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guiso N., Szatanik M., Rocancourt M. Protective activity of bordetella adenylate cyclase-hemolysin against bacterial colonization. Microb. Pathog. 1991;11:423–431. doi: 10.1016/0882-4010(91)90038-C. [DOI] [PubMed] [Google Scholar]
- 19.Guiso N., Rocancourt M., Szatanik M., Alonso J.M. Bordetella adenylate cyclase is a virulence associated factor and an immunoprotective antigen. Microb. Pathog. 1989;7:373–380. doi: 10.1016/0882-4010(89)90040-5. [DOI] [PubMed] [Google Scholar]
- 20.Khelef N., Danve B., Quentin-Millet M.J., Guiso N. Bordetella pertussis and bordetella parapertussis: Two immunologically distinct species. Infect. Immun. 1993;61:486–490. doi: 10.1128/iai.61.2.486-490.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sebo P., Glaser P., Sakamoto H., Ullmann A. High-level synthesis of active adenylate cyclase toxin of Bordetella pertussis in a reconstructed escherichia coli system. Gene. 1991;104:19–24. doi: 10.1016/0378-1119(91)90459-O. [DOI] [PubMed] [Google Scholar]
- 22.Bart M.J., Harris S.R., Advani A., Arakawa Y., Bottero D., Bouchez V., Cassiday P.K., Chiang C.S., Dalby T., Fry N.K., et al. Global population structure and evolution of Bordetella pertussis and their relationship with vaccination. mBio. 2014;5 doi: 10.1128/mBio.01074-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linz B., Ivanov Y.V., Preston A., Brinkac L., Parkhill J., Kim M., Harris S.R., Goodfield L.L., Fry N.K., Gorringe A.R., et al. Acquisition and loss of virulence-associated factors during genome evolution and speciation in three clades of bordetella species. BMC Genom. 2016;17:767. doi: 10.1186/s12864-016-3112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Havlicek V., Higgins L., Chen W., Halada P., Sebo P., Sakamoto H., Hackett M. Mass spectrometric analysis of recombinant adenylate cyclase toxin from Bordetella pertussis strain 18323/phsp9. J. Mass Spectrom. 2001;36:384–391. doi: 10.1002/jms.139. [DOI] [PubMed] [Google Scholar]
- 25.Brinig M.M., Register K.B., Ackermann M.R., Relman D.A. Genomic features of bordetella parapertussis clades with distinct host species specificity. Genome Biol. 2006;7:R81. doi: 10.1186/gb-2006-7-9-r81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouchez V., Brun D., Dore G., Njamkepo E., Guiso N. Bordetella parapertussis isolates not expressing pertactin circulating in france. Clin. Microbiol. Infect. 2011;17:675–682. doi: 10.1111/j.1469-0691.2010.03303.x. [DOI] [PubMed] [Google Scholar]
- 27.Bouchez V., Guiso N. Bordetella pertussis, B. parapertussis, vaccines and cycles of whooping cough. Pathog. Dis. 2015;73 doi: 10.1093/femspd/ftv055. [DOI] [PubMed] [Google Scholar]
- 28.Diavatopoulos D.A., Cummings C.A., Schouls L.M., Brinig M.M., Relman D.A., Mooi F.R. Bordetella pertussis, the causative agent of whooping cough, evolved from a distinct, human-associated lineage of B. bronchiseptica. PLoS Pathog. 2005;1:e45. doi: 10.1371/journal.ppat.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouchez V., Brun D., Cantinelli T., Dore G., Njamkepo E., Guiso N. First report and detailed characterization of B. pertussis isolates not expressing pertussis toxin or pertactin. Vaccine. 2009;27:6034–6041. doi: 10.1016/j.vaccine.2009.07.074. [DOI] [PubMed] [Google Scholar]
- 30.Park J., Zhang Y., Buboltz A.M., Zhang X., Schuster S.C., Ahuja U., Liu M., Miller J.F., Sebaihia M., Bentley S.D., et al. Comparative genomics of the classical bordetella subspecies: The evolution and exchange of virulence-associated diversity amongst closely related pathogens. BMC Genom. 2012;13:545. doi: 10.1186/1471-2164-13-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalet K., Weber C., Guillemot L., Njamkepo E., Guiso N. Characterization of adenylate cyclase-hemolysin gene duplication in a Bordetella pertussis isolate. Infect. Immun. 2004;72:4874–4877. doi: 10.1128/IAI.72.8.4874-4877.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chenal-Francisque V., Caro V., Boursaux-Eude C., Guiso N. Genomic analysis of the adenylate cyclase-hemolysin C-terminal region of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Res. Microbiol. 2009;160:330–336. doi: 10.1016/j.resmic.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Zaretzky F.R., Gray M.C., Hewlett E.L. Mechanism of association of adenylate cyclase toxin with the surface of Bordetella pertussis: A role for toxin-filamentous haemagglutinin interaction. Mol. Microbiol. 2002;45:1589–1598. doi: 10.1046/j.1365-2958.2002.03107.x. [DOI] [PubMed] [Google Scholar]
- 34.Perez Vidakovics M.L., Lamberti Y., van der Pol W.L., Yantorno O., Rodriguez M.E. Adenylate cyclase influences filamentous haemagglutinin-mediated attachment of Bordetella pertussis to epithelial alveolar cells. FEMS Immunol. Med. Microbiol. 2006;48:140–147. doi: 10.1111/j.1574-695X.2006.00136.x. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman C., Eby J., Gray M., Heath Damron F., Melvin J., Cotter P., Hewlett E. Bordetella adenylate cyclase toxin interacts with filamentous haemagglutinin to inhibit biofilm formation in vitro. Mol. Microbiol. 2017;103:214–228. doi: 10.1111/mmi.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouchez V., Hegerle N., Strati F., Njamkepo E., Guiso N. New data on vaccine antigen deficient Bordetella pertussis isolates. Vaccines. 2015;3:751–770. doi: 10.3390/vaccines3030751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hegerle N., Guiso N. Antibody-mediated inhibition of Bordetella pertussis adenylate cyclase-haemolysin-induced macrophage cytotoxicity is influenced by variations in the bacterial population. Microbiology. 2014;160:962–969. doi: 10.1099/mic.0.074690-0. [DOI] [PubMed] [Google Scholar]
- 38.Ramjeet M., Cox A.D., Hancock M.A., Mourez M., Labrie J., Gottschalk M., Jacques M. Mutation in the lps outer core biosynthesis gene, galu, affects lps interaction with the rtx toxins apxi and apxii and cytolytic activity of actinobacillus pleuropneumoniae serotype 1. Mol. Microbiol. 2008;70:221–235. doi: 10.1111/j.1365-2958.2008.06409.x. [DOI] [PubMed] [Google Scholar]
- 39.Bauer M.E., Welch R.A. Pleiotropic effects of a mutation in rfac on escherichia coli hemolysin. Infect. Immun. 1997;65:2218–2224. doi: 10.1128/iai.65.6.2218-2224.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novotny P., Chubb A.P., Cownley K., Montaraz J.A. Adenylate cyclase activity of a 68,000-molecular-weight protein isolated from the outer membrane of Bordetella bronchiseptica. Infect. Immun. 1985;50:199–206. doi: 10.1128/iai.50.1.199-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Welch R.A. Rtx toxin structure and function: A story of numerous anomalies and few analogies in toxin biology. Curr. Top. Microbiol. Immunol. 2001;257:85–111. doi: 10.1007/978-3-642-56508-3_5. [DOI] [PubMed] [Google Scholar]
- 42.Caroff M., Aussel L., Zarrouk H., Martin A., Richards J.C., Therisod H., Perry M.B., Karibian D. Structural variability and originality of the bordetella endotoxins. J. Endotoxin Res. 2001;7:63–68. doi: 10.1177/09680519010070011101. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X., Goebel E.M., Rodriguez M.E., Preston A., Harvill E.T. The O antigen is a critical antigen for the development of a protective immune response to Bordetella parapertussis. Infect. Immun. 2009;77:5050–5058. doi: 10.1128/IAI.00667-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El Hamidi A., Novikov A., Karibian D., Perry M.B., Caroff M. Structural characterization of Bordetella parapertussis lipid A. J. Lipid Res. 2009;50:854–859. doi: 10.1194/jlr.M800454-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hegerle N., Paris A.S., Brun D., Dore G., Njamkepo E., Guillot S., Guiso N. Evolution of french Bordetella pertussis and Bordetella parapertussis isolates: Increase of bordetellae not expressing pertactin. Clin. Microbiol. Infect. 2012;18:E340–E346. doi: 10.1111/j.1469-0691.2012.03925.x. [DOI] [PubMed] [Google Scholar]
- 46.Stainer D.W., Scholte M.J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J. Gen. Microbiol. 1970;63:211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- 47.Ladant D., Brezin C., Alonso J.M., Crenon I., Guiso N. Bordetella pertussis adenylate cyclase. Purification, characterization, and radioimmunoassay. J. Biol. Chem. 1986;261:16264–16269. [PubMed] [Google Scholar]
- 48.Weber C., Boursaux-Eude C., Coralie G., Caro V., Guiso N. Polymorphism of Bordetella pertussis isolates circulating for the last 10 years in France, where a single effective whole-cell vaccine has been used for more than 30 years. J. Clin. Microbiol. 2001;39:4396–4403. doi: 10.1128/JCM.39.12.4396-4403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cox J., Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 50.Cox J., Neuhauser N., Michalski A., Scheltema R.A., Olsen J.V., Mann M. Andromeda: A peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 2011;10:1794–1805. doi: 10.1021/pr101065j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.