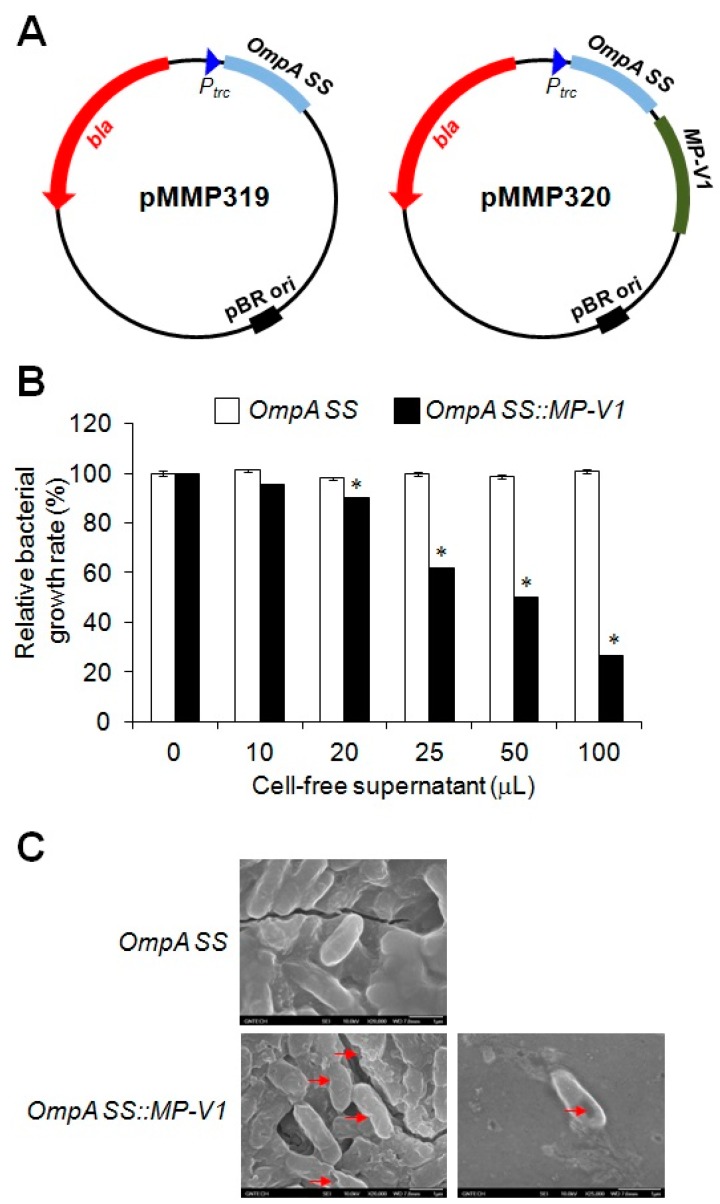
Figure 4.
Examination of antimicrobial activity with the OmpA SS::MP-V1 secretion system. (A) Construction of an E. coli secretion system for the production of MP-V1. The MP-V1 sequence was designed to be directly fused with the OmpA SS, connecting to the translational start-site derived from the Ptrc promoter. The OmpA SS, fused directly to the one from the Ptrc promoter without the MP-V1, was designed to be used as a negative control. The designed artificial genes were cloned into the T-vector, finally resulting in the pMMP319 (left) and pMMP320 (right), respectively. (B) Comparison of antimicrobial activity of the OmpA SS::MP-V1 secretion system with that of the OmpA SS one. Antimicrobial activity of the cell-free supernatants from the OmpA SS::MP-V1 strain, an E. coli cell harboring pMMP320, and the OmpA SS strain, an E. coli cell harboring pMMP319, was examined with 10 to 100 μL doses against 106 cfu/mL of Salmonella Typhimurium. Data are means ± SE (n = 3). Asterisks indicate significant effect of the OmpA SS::MP-V1 strain as compared to the OmpA SS one by the two-way ANOVA/Duncan (p < 0.05). (C) Scanning-electron micrographs of Salmonella Typhimurium treated with the cell-free supernatants from the OmpA SS::MP-V1 strain and the OmpA SS one. The red arrows indicate the pores forming into the Salmonella membrane.