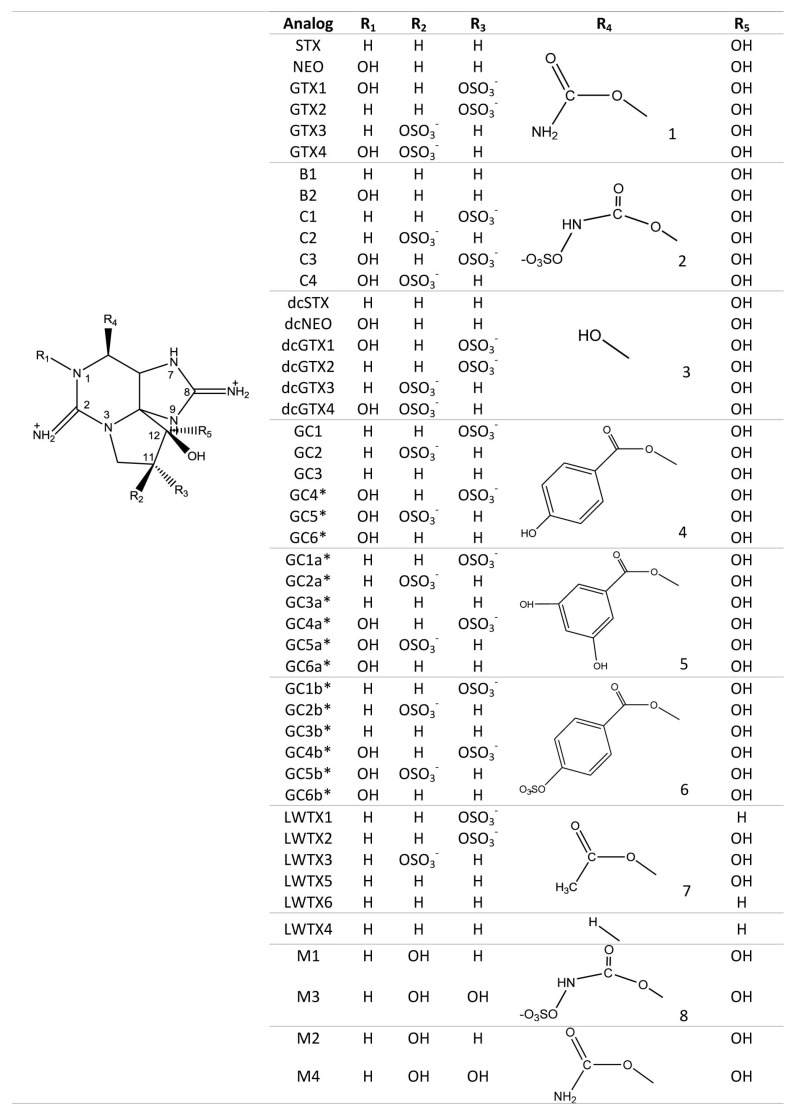
Figure 2.
Saxitoxin and major naturally-occurring analogs produced among marine dinoflagellates, various freshwater and brackish water cyanobacteria (e.g., LWTX1-LWTX6 from Lyngbya) and metabolic transformation products (e.g., M-toxins) found in mussels (Mytilus). Left: the 3,4,6-trialkyl tetrahydropurine skeleton with two guanidinium groups, common to all STX analogs. STX, saxitoxin; NEO, neosaxitoxin; GTX1-4, gonyautoxins 1 to 4; B1, B2, toxins B1 and B2; C1-C4, toxins C1 to C4; dcSTX, decarbamoyl saxitoxin; dcNEO, decarbamoyl neosaxitoxin, dcGTX1-4, decarbamoyl gonyautoxins 1 to 4; LWTX1-6, lyngbyatoxins 1 to 6; M1-M4, Mytilus toxins 1 to 4. Asterisks* for the GC-toxins refer to putative structures determined primarily by LC-MS/MS, with NMR support in some cases, but which remain to be confirmed.