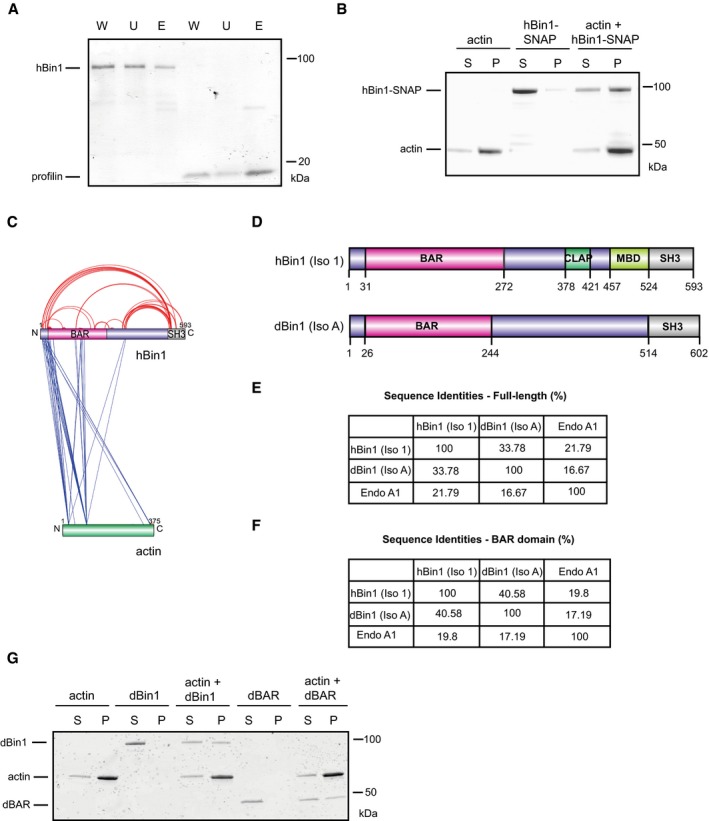
hBin1 isoform 1 binds slightly to G‐actin. G‐actin‐coupled Sepharose beads were incubated with hBin1 and profilin (both 1.25 μM) and incubated for 1 h at RT before separating them from unbound protein via centrifugation at 6,000 g for 4 min. Fractions were resolved by SDS–PAGE followed by Coomassie Blue staining. W, whole input fraction; U, unbound fraction; E, eluted fraction.
hBin1‐SNAP (isoform 1; 1 μM) binds directly to 4 μM F‐actin. Co‐sedimentation assay resolved by SDS–PAGE followed by Coomassie Blue staining.
Xvis‐visualization of crosslink sites identified for the complex of hBin1 (isoform 1) with F‐actin. Only the crosslinks with a score of 80 and higher are displayed. Intra‐molecular crosslinks are indicated in red, whereas inter‐molecular crosslinks are indicated in blue.
Domain overviews of hBin1 (isoform 1) and the Drosophila orthologue of Bin1 isoform A [dBin1 (isoform A)]. Both isoforms share the N‐terminal BAR domain and the C‐terminal SH3 domain, but differ in the middle domains.
Sequence identities of the full‐length proteins hBin1 (isoform 1), dBin1 (isoform A), and the N‐BAR family member endophilin A1 (Endo A1) analyzed with Clustal omega. Gaps in sequences were not considered.
Sequence identities of the BAR domains including the amphipathic helix of hBin1 (isoform 1), dBin1 (isoform A), and Endo A1 analyzed with Clustal omega. Gaps in sequences were not considered.
dBin1 (isoform A) and its BAR domain (dBAR; both 1 μM) bind directly to 4 μM F‐actin. Co‐sedimentation assay resolved by SDS–PAGE followed by Coomassie Blue staining.