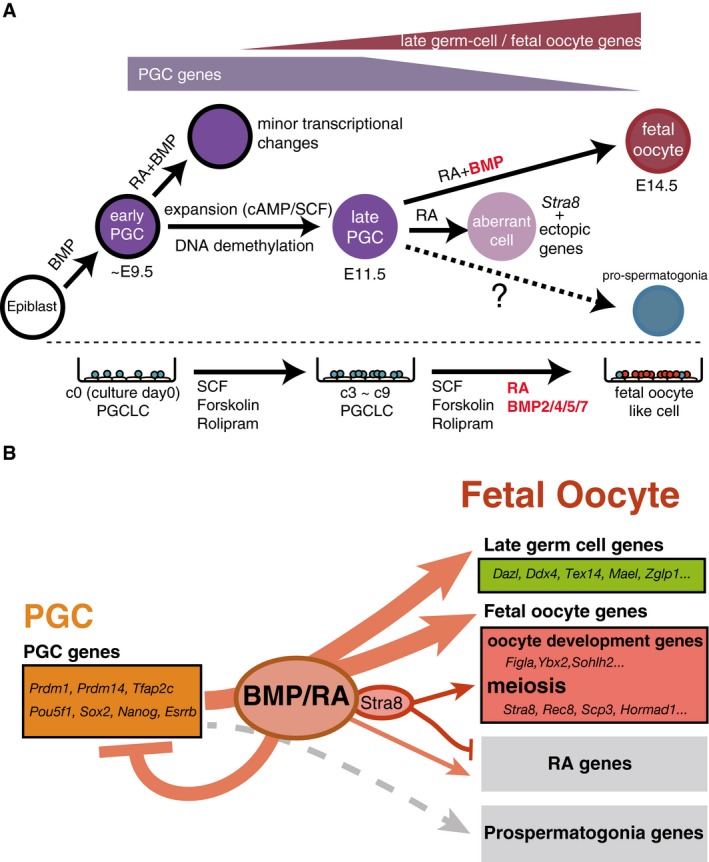
A model for the roles of BMP and RA signaling. BMP and RA signaling contribute to the repression of early PGC genes (e.g., Prdm1, Prdm14, Tfap2c, Pou5f1, Sox2, Nanog, and Esrrb) and to the up‐regulation of late germ‐cell genes (e.g., Ddx4, Dazl, Piwil2, Mov10l1, and Mael) and fetal oocyte genes (e.g., Stra8, Rec8, Sycp3, Hormad1 as meiosis genes, and Figla, Ybx2, Sohlh2 as oocyte‐development genes). With BMP signaling, STRA8 promotes the expression of meiosis genes and inhibits the ectopic expression of developmental genes induced by RA signaling (RA genes). STRA8 does not have a significant impact on late germ‐cell genes and oocyte‐development genes. Without BMP signaling, STRA8 cannot fully up‐regulate meiosis genes or induce meiosis entry. BMP and RA signaling do not up‐regulate prospermatogonia genes.