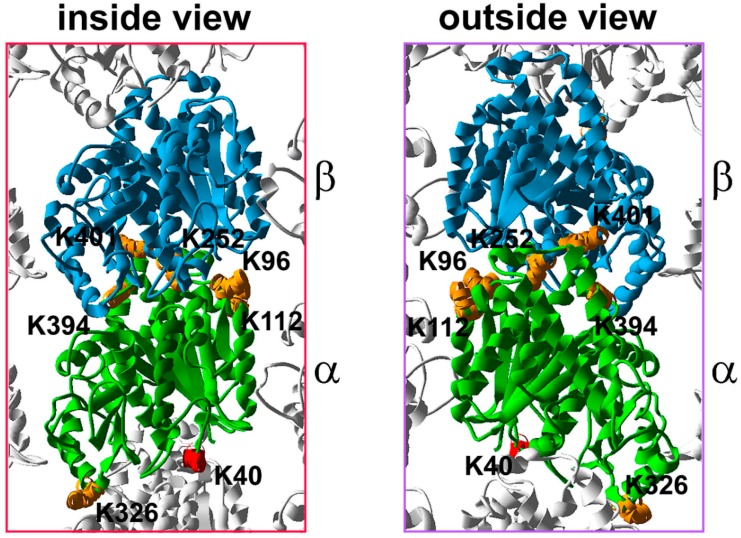
Figure 2.
The distribution of the acetylated residues within the α,β-tubulin heterodimer. 3D model of a tubulin heterodimer within the microtubule lattice based on 3J6F.pdb (NCBI database), an inside and outside view. Putative acetylation sites are marked in orange. An approximate location of K40 is marked in red (K40 was not included in the original 3D model). α-Tubulin K96 is located within the S3 strand and builds the plus end surface, responsible for the interactions with β-tubulin of the same heterodimer. α-Tubulin K112 is located in close vicinity to the H3 surface while K326 is located within the H10 helix, and builds the minus end surface responsible for longitudinal interactions between heterodimers within the same protofilament or with γ-tubulin at the nucleation site. α-Tubulin K394 and K401 are located within the H11 helix and H11–H12 loop, respectively which are exposed on the outside surface involved in the interactions with non-tubulin partners of tubulin. K252 of β-tubulin is located within the H8 helix and builds the minus end surface.