Table 2. Optimization of the enantioselective aminocyclopropane activation a .
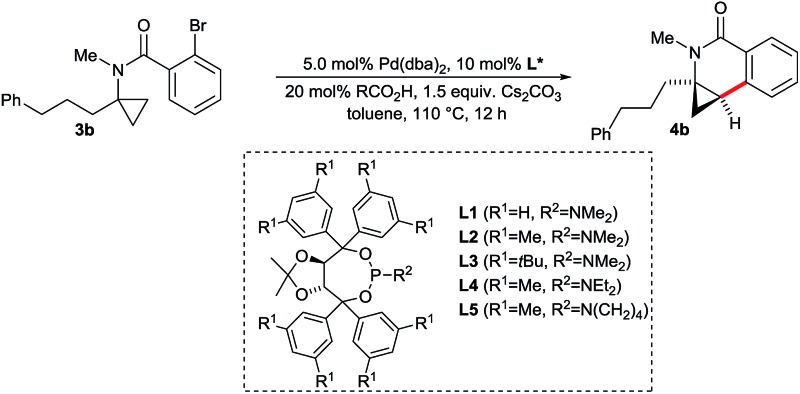
| ||||
| Entry | L* | RCO2H | % yield of 4b b | er c |
| 1 | L1 | AdCO2H | 2 | n.d. |
| 2 | L2 | AdCO2H | 99 | 93.5 : 6.5 |
| 3 | L3 | AdCO2H | 55 | 85.5 : 14.5 |
| 4 | L4 | AdCO2H | 94 | 90 : 10 |
| 5 | L5 | AdCO2H | 99 | 93 : 7 |
| 6 | L2 | — | 3 | n.d. |
| 7 | L2 | AcOH | 86 | 60 : 40 |
| 8 | L2 | XanthCO2H | 25 | 77 : 23 |
| 9 | L2 | PivOH | 92 | 92.5 : 7.5 |
| 10 d | L2 | AdCO2H | 99 | 93.5 : 6.5 |
| 11 e | L2 | AdCO 2 H | 96 (93) | 93.5 : 6.5 |
aReaction conditions: 0.05 mmol 3b, 5.0 mol% Pd(dba)2, 10 mol% L*, 20 mol% RCO2H, 1.5 equiv. Cs2CO3 0.1 M in toluene, 110 °C, 12 h.
bDetermined by 1H-NMR with an internal standard (isolated yield in parentheses).
cer values were determined by HPLC with a chiral stationary phase.
dWith K2CO3 instead of Cs2CO3.
eWith 2.5 mol% Pd(dba)2, 5.0 mol% L2, 0.25 M in toluene.
